��Ŀ����
(10��)���꣬�ҹ��ں�����ҵ��ȡ������������Ŀ�ijɾͣ����۷ɴ���α�����ϵ
�л������̫�ա�
(1)������������ʽ����ƽ���������(![]() )��Ϊȼ�ϣ�
)��Ϊȼ�ϣ�![]() ��Ϊ�ƽ�����
��Ϊ�ƽ�����
��![]() ����Ҫ��������ȼ�������ڹ���ʱ���������ɫ����
����Ҫ��������ȼ�������ڹ���ʱ���������ɫ����![]() ���Ի�������
���Ի�������
����Ⱦ��Ϊ������Ⱦ��ʹ������ (����ĸ)����֮��
A��Һ̬�� B��![]() C��
C��![]() D��Һ̬��
D��Һ̬��
���ڼ��������£�������![]() �ʹ������Ʒ�Ӧ������ȡ����(������
�ʹ������Ʒ�Ӧ������ȡ����(������
ͬʱ����������)����÷�Ӧ�����ӷ���ʽ�� ��
(2)�ɴ������ڿ����ĸ��¹�������ͼ��ʾ��
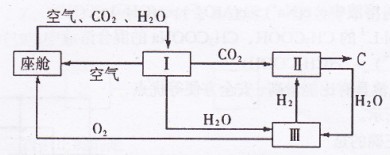
�������ڿ������¹��̿���ѭ�����õ�����Ϊ![]() ��
��![]() �� ��װ�â���
�� ��װ�â���
������Ӧ�Ļ�ѧ����ʽΪ ��
�ڴ�װ��I����ɿ���![]() ����Դ�����Աÿ������35mol
����Դ�����Աÿ������35mol![]() ��ÿ�����
��ÿ�����
�������к�18 mol![]() ��������������к�
��������������к�![]() mol��
mol��
��10�֡�ÿ��2�֡��� �� �� Դ ��
��1���� BD �� CO(NH2)2 + ClO�� + 2OH�� = N2H4 + Cl�� + CO32- + H2O
��2���� H2O��������N2�� 2H2 + CO2 = 2H2O + C �� 26

��ϰ��ϵ�д�
�����Ŀ