��Ŀ����
Ϊ�����Դ��ȱ��������Ⱦ����������о��ü״����ж�������������и�ʴ�ԣ��������������ȼ�ϣ�������״�ȼ�յ��Ȼ�ѧ����ʽ���£�
2C8H18��l��+25O2��g��=16CO2��g��+18H2O��l������H=-a kJ?mol-1
2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��l������H=-b kJ?mol-1
����Ҫ�ش��������⣺
��1����ҵ�ϳɼ״���һ��;���У������ʼ��ת����ϵ��ͼ1����֪M�Ǻ���10�����ӵķ��ӣ�XΪ���ʡ�YΪ��������ǻ�ԭ�Ե����壮��Ӧ��I���Ļ�ѧ����ʽΪ ��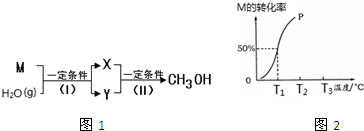
��2����2.0mol M��2.0mol H2O��g��ͨ��100L���ܱ������У���һ�������·�����Ӧ��I���������һ����ѹǿ��M��ת�������¶ȵĹ�ϵ��ͼ2��
�ټ���T1��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2O��g����ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ mol?L-1?min-1��
��T1��ʱ��Ӧ��I����ƽ�ⳣ��Ϊ ��
�۷�Ӧ��I���ġ�H 0���������������
��3���������ͻ�����ʱ����������״ȼ�ϣ��ɿ������壩������Ļ��������ѹ����ȼ�������������������У����ü״��������������ȼ�ϣ���������ͻ���
�ٸ���֮һ�ǵ������ͻ�����ϵͳ���Ե����������ȼ�������֮��ȣ�������ǰ�������ͻ��Ļ���������ͣ���״������������Ϊm��������������ͻ��Ļ�����м״�����״������������Ϊn����n m�����������������=������
������ݼ״������͵����ʲ��죬Ԥ�����ͻ���������һ�����⣬���������ͣ�
��Ҫ��������⣺ �����ͣ� ��
��4����ʹ�����͡��״�������ȼ��ʱЧ����ͬ���Թ��㣺���ͻ�����ʱ����ͬ������������״����ṩ������������֮��Ϊ ��
2C8H18��l��+25O2��g��=16CO2��g��+18H2O��l������H=-a kJ?mol-1
2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��l������H=-b kJ?mol-1
����Ҫ�ش��������⣺
��1����ҵ�ϳɼ״���һ��;���У������ʼ��ת����ϵ��ͼ1����֪M�Ǻ���10�����ӵķ��ӣ�XΪ���ʡ�YΪ��������ǻ�ԭ�Ե����壮��Ӧ��I���Ļ�ѧ����ʽΪ

��2����2.0mol M��2.0mol H2O��g��ͨ��100L���ܱ������У���һ�������·�����Ӧ��I���������һ����ѹǿ��M��ת�������¶ȵĹ�ϵ��ͼ2��
�ټ���T1��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2O��g����ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ
��T1��ʱ��Ӧ��I����ƽ�ⳣ��Ϊ
�۷�Ӧ��I���ġ�H
��3���������ͻ�����ʱ����������״ȼ�ϣ��ɿ������壩������Ļ��������ѹ����ȼ�������������������У����ü״��������������ȼ�ϣ���������ͻ���
�ٸ���֮һ�ǵ������ͻ�����ϵͳ���Ե����������ȼ�������֮��ȣ�������ǰ�������ͻ��Ļ���������ͣ���״������������Ϊm��������������ͻ��Ļ�����м״�����״������������Ϊn����n
������ݼ״������͵����ʲ��죬Ԥ�����ͻ���������һ�����⣬���������ͣ�
��Ҫ��������⣺
��4����ʹ�����͡��״�������ȼ��ʱЧ����ͬ���Թ��㣺���ͻ�����ʱ����ͬ������������״����ṩ������������֮��Ϊ
��������1������ת����ϵͼ��Ԫ���غ��֪M����̼Ԫ�أ���֪M�Ǻ���10�����ӵķ��ӣ�XΪ���ʡ�YΪ��������ǻ�ԭ�Ե����壬��MΪCH4��XΪH2��YΪCO��
��2������������ʽ���ת����H2O�����ʵ�����������䷴Ӧ���ʣ�
�������ƽ��ʱ�����ʵ�Ũ�ȣ������ƽ�ⳣ����
�۸���ͼ���֪�¶�Խ�ߣ������ת����Խ�������жϣ�
��3���ٸ���ȼ�շ��̿�֪�������ʵ��������ͺͼ״���ȫȼ�գ��������ĵ������ࣻ
�ڸ����������ʿ�֪�����ͺͼ״��ķе㲻ͬ������Ҫ��������ϵͳ��
��4���������ͬ���������ͺͼ״���ȫȼ�շų����������������ֵ��
��2������������ʽ���ת����H2O�����ʵ�����������䷴Ӧ���ʣ�
�������ƽ��ʱ�����ʵ�Ũ�ȣ������ƽ�ⳣ����
�۸���ͼ���֪�¶�Խ�ߣ������ת����Խ�������жϣ�
��3���ٸ���ȼ�շ��̿�֪�������ʵ��������ͺͼ״���ȫȼ�գ��������ĵ������ࣻ
�ڸ����������ʿ�֪�����ͺͼ״��ķе㲻ͬ������Ҫ��������ϵͳ��
��4���������ͬ���������ͺͼ״���ȫȼ�շų����������������ֵ��
����⣺��1������ת����ϵͼ��Ԫ���غ��֪M����̼Ԫ�أ���֪M�Ǻ���10�����ӵķ��ӣ�����MΪCH4��XΪ���ʡ�YΪ��������ǻ�ԭ�Ե����壬��XΪH2��YΪCO�����Է�Ӧ�Ļ�ѧ����ʽΪ��CH4+H2O
CO+3H2��
�ʴ�Ϊ��CH4+H2O
CO+3H2��
��2������μӷ�Ӧ�ļ�������ʵ���Ϊxmol��
CH4 +H2O
CO+3H2
��ʼ����mol����2 2 0 0
ת������mol����x x x 3x
ƽ������mol����2-x 2-x x 3x
��֪ƽ��ʱCH4��ת����Ϊ50%����
��100%=50%������x=1����c��H2O��=
=0.01mol/L��v��H2O��=
=
=2.0��10-3mol?L-1?min-1��
�ʴ�Ϊ��2.0��10-3��
��ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊ��c��CH4��=
=0.01mol/L��c��H2O��=
=0.01mol/L��c��CO��=
=0.01mol/L��c��H2��=
=0.03mol/L��
����ƽ�ⳣ��Ϊ��K=
=
=2.7��10-3��
�ʴ�Ϊ��2.7��10-3��
�۸���ͼ���֪�¶�Խ�ߣ������ת����Խ��˵�������¶�ƽ�����������ƶ�������������Ϊ���ȷ�Ӧ������H��0��
�ʴ�Ϊ������
��3���ٸ���ȼ�շ��̿�֪�������ʵ�����������ȫȼ����״���ȫȼ����Ƚϣ��������ĵ������࣬���Ը���ǰ�������ͻ��Ļ���������ͣ���״������������С�ڸ�����������ͻ��Ļ�����м״�����״�����������ȣ���m��n����n��m��
�ʴ�Ϊ������
�ڸ����������ʿ�֪�����ͺͼ״��ķе㲻ͬ������Ҫ��������ϵͳ���ʴ�Ϊ���������ͻ�����ϵͳ����Ϊ���ͺͼ״��ķе㲻ͬ��
��4�����ͺͼ״���������Ϊ1g�������͵����ʵ���Ϊ
mol������ȫȼ�շų�����Ϊ
��
=
KJ���״������ʵ���Ϊ
mol������ȫȼ�շų�����Ϊ
��
=
��������ͬ������������״����ṩ������������֮��Ϊ
=
��
�ʴ�Ϊ��
��
| һ�������� |
�ʴ�Ϊ��CH4+H2O
| һ�������� |
��2������μӷ�Ӧ�ļ�������ʵ���Ϊxmol��
CH4 +H2O
| һ�������� |
��ʼ����mol����2 2 0 0
ת������mol����x x x 3x
ƽ������mol����2-x 2-x x 3x
��֪ƽ��ʱCH4��ת����Ϊ50%����
| x |
| 2 |
| 1mol |
| 100L |
| ��c |
| t |
| 0.01mol/L |
| 5min |
�ʴ�Ϊ��2.0��10-3��
��ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊ��c��CH4��=
| 1mol |
| 100L |
| 1mol |
| 100L |
| 1mol |
| 100L |
| 3mol |
| 100L |
����ƽ�ⳣ��Ϊ��K=
| c(CO)?c3(H2) |
| c(CH4)?c(H2O) |
| 0.01��0.032 |
| 0.01��0.01 |
�ʴ�Ϊ��2.7��10-3��
�۸���ͼ���֪�¶�Խ�ߣ������ת����Խ��˵�������¶�ƽ�����������ƶ�������������Ϊ���ȷ�Ӧ������H��0��
�ʴ�Ϊ������
��3���ٸ���ȼ�շ��̿�֪�������ʵ�����������ȫȼ����״���ȫȼ����Ƚϣ��������ĵ������࣬���Ը���ǰ�������ͻ��Ļ���������ͣ���״������������С�ڸ�����������ͻ��Ļ�����м״�����״�����������ȣ���m��n����n��m��
�ʴ�Ϊ������
�ڸ����������ʿ�֪�����ͺͼ״��ķе㲻ͬ������Ҫ��������ϵͳ���ʴ�Ϊ���������ͻ�����ϵͳ����Ϊ���ͺͼ״��ķе㲻ͬ��
��4�����ͺͼ״���������Ϊ1g�������͵����ʵ���Ϊ
| 1 |
| 114 |
| a |
| 2 |
| 1 |
| 114 |
| a |
| 228 |
| 1 |
| 32 |
| b |
| 2 |
| 1 |
| 32 |
| b |
| 64 |
| ||
|
| 16a |
| 57b |
�ʴ�Ϊ��
| 16a |
| 57b |
���������⿼�������ʵ��ƶϡ�����ʽ����д����ѧƽ����йؼ��㡢ƽ�ⳣ������Ӧ�ȵļ���ȣ��漰��֪ʶ��϶࣬��Ŀ�ѶȽϴ��ضԻ���֪ʶ��Ӧ�õĿ��飬�Լ���ѧ���ۺ������Ŀ��飮

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ
 ��______�����ѧ��ʴ���绯ѧ��ʴ�����Ǹ����������ձ�ĸ�ʴ��
��______�����ѧ��ʴ���绯ѧ��ʴ�����Ǹ����������ձ�ĸ�ʴ��