��Ŀ����
����ͼ��A��B��C��D��E��F��G����ʾһ�ַ�Ӧ�������������ķ�Ӧ�����������ԣ���������������������������ϵ����������A��B��C��D��E��F��G�������������D��G�����Σ���A��B��C��D��ͬһԪ�أ���A��E��F��G��ͬһԪ�أ���FΪ���ʣ���E��������������B��F��������ˮ��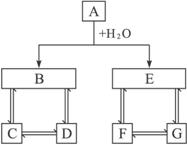
��1��д���������ʵĻ�ѧʽ��A____________��D____________��E____________��G____________��
��2��д�����з�Ӧ�����ӷ���ʽ��
��B��D____________________________________��
��D��C____________________________________��
��C+D��B____________________________________��
��1��Al2S3 NaAlO2 H2S Na2S?
��2����Al��OH��3+OH-====![]() +2H2O ��
+2H2O ��![]() +4H+====Al3++2H2O
+4H+====Al3++2H2O
��Al3++3![]() +6H2O====4Al��OH��3��
+6H2O====4Al��OH��3��
�����������ͻ�ƿ�ΪA��H2O��B��E����ˮ�ܷ�Ӧ�����������ʵ��У�2Na+2H2O====2NaOH+H2����2Na2O2+2H2O====4NaOH+O2����Mg3N2+6H2O====3Mg��OH��2��+2NH3����CaC2+2H2O��Ca��OH��2+CH��CH����Al2S3+6H2O====2Al��OH��3��+3H2S�����ٽ�ϡ�A��E��F��G��ͬһԪ�أ���FΪ���ʡ�������Ϣ��֪��EΪH2S��FΪS��������֪AΪAl2S3��DΪNaAlO2��GΪNa2S��
��Ӧ�����ӷ���ʽΪ����Al��OH��3+OH-====![]() +2H2O��
+2H2O��
��![]() +4H+====Al3++2H2O����Al3++3
+4H+====Al3++2H2O����Al3++3![]() +6H2O====4Al��OH��3����
+6H2O====4Al��OH��3����

 ��У����ϵ�д�
��У����ϵ�д�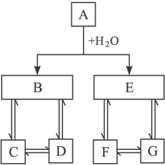
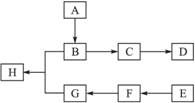
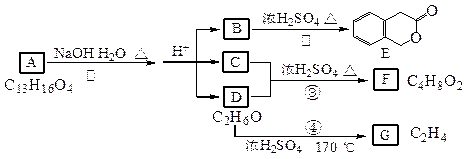
 _______________;
_______________;
 ȷ���� __
ȷ���� __