��Ŀ����
������������ѧ���и���ѡһ������
1�ۻ�ѧ�������ʽṹ�����ʣ�Q��R��X��Y��Z����Ԫ�ص�ԭ���������ε�������֪��
��Z��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ�
��Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�Ϊmsnmpn;
��Rԭ�Ӻ���L�������Ϊ������
��Q��Xԭ��p����ĵ������ֱ�Ϊ2��4��
��ش��������⣺
��1��Z2+�ĺ�������Ų�ʽ��___________��
��2���ڣ�Z��NH3��4��2+�����У�Z2+�Ŀչ������NH3�����ṩ��_________�γ���λ����
��3��Q��Y�γɵ������̬�⻯��ֱ�Ϊ�ס��ң������ж���ȷ����_________��
a.�ȶ��ԣ��ף��ң��е㣺�ף��� b.�ȶ��ԣ��ף��ң��е㣺�ף���
c.�ȶ��ԣ��ף��ң��е㣺�ף��� d.�ȶ��ԣ��ף��ң��е㣺�ף���
��4��Q��R��Y����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ_________����Ԫ�ط������𣩡�
��5��Q��һ���⻯����Է�������Ϊ26��������ЦҼ���м��ļ���֮��Ϊ_________��
��6������Ԫ���У��縺���������С�����ַǽ���Ԫ���γɵľ�������_________��������ͣ���
2�ۻ�ѧ�����л���ѧ�������л���A�dz��õ�ʳ���Ϳ�������������ʽΪC10H12O5���ɷ�������ת����

��֪B����Է�������Ϊ60��������ֻ��һ������C�Ľṹ�ɱ�ʾΪ�� 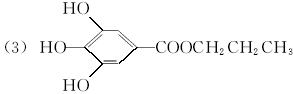 �����У���X����Y��Ϊ�����ţ���
�����У���X����Y��Ϊ�����ţ���
��ش��������⣺
��1������ϵͳ��������B������Ϊ________________��
��2�������š�X������Ϊ____________���߾���E������Ϊ____________��
��3��A�Ľṹ��ʽΪ________________________��
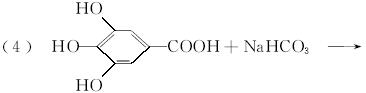
��4����Ӧ�ݵĻ�ѧ����ʽΪ____________________________________��
��5��C�ж���ͬ���칹�壬д������2�ַ�������Ҫ���ͬ���칹��Ľṹ��ʽ��
��.���б��� ��.�ܷ���������Ӧ ��.���ܷ���ˮ�ⷴӦ
��6���ӷ��ӽṹ�Ͽ���A���п��������õ���Ҫԭ����____________������ţ���
a.���б��� b.�����ʻ� c.���з��ǻ�
1(1)1s22s22p63s23p63d9
(2)�¶Ե��ӣ��µ��Ӷԣ�
��3��b
(4)Si��C��N
(5)3��2
(6)ԭ�Ӿ���
���������ҵ�ԭ������Ϊ29������ΪCuԪ�أ��ۺϢڢܷۢ�����֪Q��R��X��Y�ֱ�ΪC��N��O��Si������Ԫ�������ɡ���ѧ�������ۼ�������ṹ��֪ʶ�����С�ⲻ������
2��1��1-����
��2���Ȼ� 

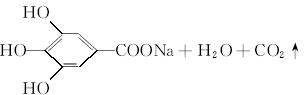
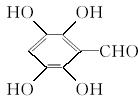
(5)
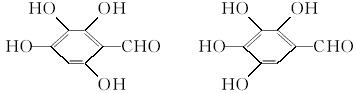
д������3���ṹ��ʽ�е�����2��
��6��c
��������C�Ļ�ѧ���ʿ�֪��C�����к��б��������ǻ����Ȼ������A�����е���ԭ������֪C�ķ��ӽṹ�У���XΪ��COOH����YΪ��OH����A�����ʷ�����BΪ��������Է�������Ϊ60�����ӽṹ����һ�������ɵ�B�Ľṹ��ʽΪCH3CH2CH2OH�������Ͻ������д��A��E��C�Ľṹ��ʽ��C��ͬ���칹���У�����������һ������һ����CHO��4����OH���ɴ�֪������ֻ��һ��̼ԭ������һ��Hԭ�ӣ���Hԭ���롪CHO���ڡ��䡢�����ֲ�ͬ��λ�ã�д�����е��������ּ��ɡ��������з��ǻ������п��������ã�����A�Ŀ��������������ڷ��ǻ���

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�