��Ŀ����
����Ŀ�����Ǻ�ˮ�к�����ḻ��Ԫ�أ��ȵĵ��ʼ��仯���������ɡ���������Ӧ�ù㷺��
��1������ˮ������Һ�Ƚ���ɱ����������������ˮ�����Ŀ��淴ӦΪ______________�������ӷ���ʽ��ʾ����
��2����Һ��������������л��ȴ��Σ�����彡��������ʹ�ö������ȣ�ClO2������Һ�ȡ���ҵ���Ի�����FeS2���������ƣ�NaClO3����������Һ����Ʊ������������塣��֪�������е���Ԫ�أ�-1�ۣ�����������SO42-��д���Ʊ��������ȵ����ӷ���ʽ_____________________________________��
��3������Ư�۹�������Ҫ�豸���Ȼ����������ϵ��·�Ϊ�IJ㣬��ͼΪ��������ʾ��ͼ��
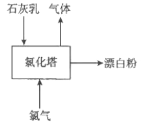
����Ư�۷�Ӧ�Ļ�ѧ����ʽΪ_____________________��ʵ�������У���ʯ���飨����3%-6%ˮ�ֵ���ʯ�ң��������������£�������������ײ�ͨ�롣�������ϵ�Ŀ����_____________________________��
��4����Ī�������Բⶨ��Һ��Cl-�ĺ�����Ī������һ�ֳ����ζ������ñ�AgNO3��Һ�ζ�����Һ����K2CrO4Ϊָʾ�����ζ��յ����������Һ�г���ש��ɫ������Ag2CrO4������֪ƽ��Cr2O72-+H2O![]() 2CrO42-+2H+���õζ�������Ҫ����pH��Χ��6.5~10.5����pHС��6.5��ʹ�ⶨ���ƫ�ߡ����ƽ���ƶ�ԭ������ƫ�ߵ�ԭ��:_______________________________ ��
2CrO42-+2H+���õζ�������Ҫ����pH��Χ��6.5~10.5����pHС��6.5��ʹ�ⶨ���ƫ�ߡ����ƽ���ƶ�ԭ������ƫ�ߵ�ԭ��:_______________________________ ��
���𰸡�Cl2+H2O![]() H++Cl-+HClO 15ClO3-+FeS2+14H+=15ClO2+Fe3++2SO42-+7H2O 2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O �ٽ���Ӧ���ij�ֽӴ�����߷�Ӧ��Ч�� ���������Ũ�ȣ�ƽ�������ƶ���CrO42-Ũ�Ƚ��ͣ��������ɵ�Ag2CrO4��ש��ɫ����������������Ũ���������ĵ���������������ʹ��õ������Ӻ���ƫ��
H++Cl-+HClO 15ClO3-+FeS2+14H+=15ClO2+Fe3++2SO42-+7H2O 2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O �ٽ���Ӧ���ij�ֽӴ�����߷�Ӧ��Ч�� ���������Ũ�ȣ�ƽ�������ƶ���CrO42-Ũ�Ƚ��ͣ��������ɵ�Ag2CrO4��ש��ɫ����������������Ũ���������ĵ���������������ʹ��õ������Ӻ���ƫ��
��������
��1��������ˮ�ķ�ӦΪ��Cl2+H2O![]() HCl+HClO����д�����ӷ���ʽ���ɽ��
HCl+HClO����д�����ӷ���ʽ���ɽ��
��2���������Ϣ��֪�÷�Ӧ��������ΪNaClO3����ԭ��ΪFeS2����������ΪSO42-��Fe3+����ԭ����ΪClO2������������ԭ��Ӧ����ʽ����ƽ�������
��3��������ʯ����ķ�Ӧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O����ҵ�Ϲ���������ѡ����������߲��ʺ����Ч������������з������ɴ˿ɵý��ۡ�
��4������Һ��pH��С����������Ũ��������ƽ��Cr2O72-+H2O![]() 2CrO42-+2H+�����ƶ�����Һ��CrO42-Ũ��ƫС����Ag2CrO4���ܶȻ��ɵ����ɵ�Ag2CrO4��ש��ɫ����������������Ũ���������ĵ��������������࣬�Ӷ�ʹ��õ������Ӻ���ƫ�ߣ��ݴ˷����ɵý��ۡ�
2CrO42-+2H+�����ƶ�����Һ��CrO42-Ũ��ƫС����Ag2CrO4���ܶȻ��ɵ����ɵ�Ag2CrO4��ש��ɫ����������������Ũ���������ĵ��������������࣬�Ӷ�ʹ��õ������Ӻ���ƫ�ߣ��ݴ˷����ɵý��ۡ�
��1����������ˮ�����в���������ˮ��Ӧ����HCl��HClO��������Ϊ���ᣬ��Һ����Ҫ�Է�����ʽ���ڣ��ʴ�Ϊ��Cl2+H2O![]() H++Cl-+HClO��
H++Cl-+HClO��
��2���������Ϣ��֪�÷�Ӧ��������ΪNaClO3����ԭ��ΪFeS2����������ΪSO42-��Fe3+����ԭ����ΪClO2������������ԭ��Ӧ���ɿɵ÷�Ӧ�����ӷ���ʽΪ��15ClO3-+FeS2+14H+=15ClO2+Fe3++2SO42-+7H2O���ʴ�Ϊ��15ClO3-+FeS2+14H+=15ClO2+Fe3++2SO42-+7H2O��
��3��������ʯ���鷴Ӧ�����Ȼ��ơ�������ƺ�ˮ����ѧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O����ҵ��ͨ����ȡ��������[��ʯ���飨����3%-6%ˮ�ֵ���ʯ�ң��������������£�������������ײ�ͨ��]���ٽ���Ӧ���ij�ֽӴ�����߷�Ӧ��Ч�ʣ��ʴ�Ϊ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O���ٽ���Ӧ���ij�ֽӴ�����߷�Ӧ��Ч�ʣ�
��4������Һ��pHС��6.5����������Ũ��������ƽ��Cr2O72-+H2O![]() 2CrO42-+2H+�����ƶ�����Һ��CrO42-Ũ��ƫС����Ag2CrO4���ܶȻ��ɵ����ɵ�Ag2CrO4��ש��ɫ����������������Ũ���������ĵ��������������࣬�Ӷ�ʹ��õ������Ӻ���ƫ�ߣ��ʴ�Ϊ�����������Ũ�ȣ�ƽ�������ƶ���CrO42-Ũ�Ƚ��ͣ��������ɵ�Ag2CrO4��ש��ɫ����������������Ũ�����������������������࣬ʹ��õ������Ӻ���ƫ�ߡ�
2CrO42-+2H+�����ƶ�����Һ��CrO42-Ũ��ƫС����Ag2CrO4���ܶȻ��ɵ����ɵ�Ag2CrO4��ש��ɫ����������������Ũ���������ĵ��������������࣬�Ӷ�ʹ��õ������Ӻ���ƫ�ߣ��ʴ�Ϊ�����������Ũ�ȣ�ƽ�������ƶ���CrO42-Ũ�Ƚ��ͣ��������ɵ�Ag2CrO4��ש��ɫ����������������Ũ�����������������������࣬ʹ��õ������Ӻ���ƫ�ߡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�