��Ŀ����
��NAΪ�����ӵ�������ֵ����������������� �� ����
| A����״���£�22.4 L SO2��O2�Ļ�������к��е���ԭ����Ϊ2NA |
| B����״���£�22.4 L��ϩ�к��й��õ��ӶԵ���ĿΪ6NA |
| C���ܱ������У�3 mol H2��1 mol N2�ڸ��¡���ѹ�������������³�ַ�Ӧ�������ڵ������������Ϊ2NA |
| D��2.8 g��CO��2.8 g��N2������������Ϊ1.4NA |
C
SO2��O2�����ж���������ԭ�ӣ�A��ȷ����1����ϩ�����к���6�Թ��õ��ӶԿ�֪��B��ȷ����H2��N2�����������·����ķ�Ӧ�ǿ��淴Ӧ����3 mol H2��1 mol N2������ȫ��Ӧ����2 mol NH3����C����CO��N2��Ħ��������ȣ���Ϊ28 g��mol��1,2.8 g CO��2.8 g N2����������Ҳ��ȣ���Ϊ1.4NA��D��ȷ��

��ϰ��ϵ�д�
 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ
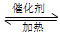 2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g)����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���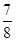 ����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��
����N2��ת����Ϊ________����NH3��Ũ�ȱ仯��ʾ�ù��̵ķ�Ӧ����Ϊ________��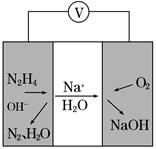
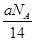
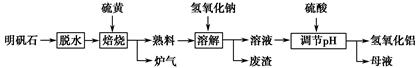
 Al2��SO4��3��
Al2��SO4��3��
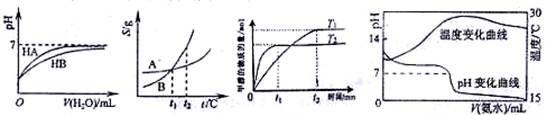
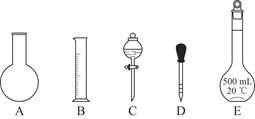
 NA,�����и�������ȵ��ǣ��� ����
NA,�����и�������ȵ��ǣ��� ����