��Ŀ����
��ʡ����ط�����ʯ��ʯ���кܶ���ʯ������ʯ�ҵ���ҵ������ʯ��ʱ����800��������ܱյ�ʯ��Ҥ�ڴ�������ƽ�⣺CaCO3(s) CaO(s)+CO2(g)��ƽ�ⳣ��K=c(CO2)=0.003
CaO(s)+CO2(g)��ƽ�ⳣ��K=c(CO2)=0.003
(1)��CO2(g)��CaCO3(s)��CaO(s)������A��B���ֲ�ͬ��Ͷ�Ϸ�ʽ������һ��10 L�ܱ�������
 CaO(s)+CO2(g)��ƽ�ⳣ��K=c(CO2)=0.003
CaO(s)+CO2(g)��ƽ�ⳣ��K=c(CO2)=0.003 (1)��CO2(g)��CaCO3(s)��CaO(s)������A��B���ֲ�ͬ��Ͷ�Ϸ�ʽ������һ��10 L�ܱ�������
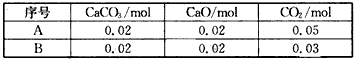
�����㹻��ʱ����ά���¶Ȳ��䣬��A��Ͷ�Ϸ�ʽ��������CaCO3(s)�����ʵ���Ϊ____mol����B��Ͷ�Ϸ�ʽ��������CaCO3(s)�����ʵ���Ϊ____mol��
(2)������ɺ�����������Ҫ�ɷֶ���̼��ƣ�������ʯ��(CaSO4��2H2O)��ԭ���Ƴ���һ�ֺ�ɫ�ġ�ɺ����������ðɺ�����ۡ������һ����ʵ�鷽���������ɺ����____��
(3)��¯ˮ���к��н�������ˮ�ֽ����������CaSO4��������̼������Һ������ʹ֮ת��Ϊ���ɡ�������������ʣ���ת���Ļ�ѧ��Ӧ����ʽ��____________________��
(2)������ɺ�����������Ҫ�ɷֶ���̼��ƣ�������ʯ��(CaSO4��2H2O)��ԭ���Ƴ���һ�ֺ�ɫ�ġ�ɺ����������ðɺ�����ۡ������һ����ʵ�鷽���������ɺ����____��
(3)��¯ˮ���к��н�������ˮ�ֽ����������CaSO4��������̼������Һ������ʹ֮ת��Ϊ���ɡ�������������ʣ���ת���Ļ�ѧ��Ӧ����ʽ��____________________��
(1)0. 04��0.02
(2)ȡ��������ɺ��������һ��ϡ���ᣬ�������ݲ���ɺ����Ϊ�棬����Ϊ��
(3)CaSO4(s)+Na2CO3(aq) CaCO3(s)+Na2SO4 (aq) (��CaSO4+Na2CO3=CaCO3+Na2SO4)
CaCO3(s)+Na2SO4 (aq) (��CaSO4+Na2CO3=CaCO3+Na2SO4)
(2)ȡ��������ɺ��������һ��ϡ���ᣬ�������ݲ���ɺ����Ϊ�棬����Ϊ��
(3)CaSO4(s)+Na2CO3(aq)
 CaCO3(s)+Na2SO4 (aq) (��CaSO4+Na2CO3=CaCO3+Na2SO4)
CaCO3(s)+Na2SO4 (aq) (��CaSO4+Na2CO3=CaCO3+Na2SO4) 
��ϰ��ϵ�д�
 ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�
�����Ŀ