��Ŀ����
��12�֣��ҹ�������ɽ���������ׯ����������δ���κδ����ĵر�ˮΪ������Щ�ر�ˮ�ܵ���ͬ�̶ȵ���Ⱦ����ɽ��Ⱥ�ڵ����彡����ɲ���Ӱ�졣Ŀǰ����Ժ��������Ͷ������ʽ𣬲�ȡ������ʩΪ��Щ��ׯ��ˮ������װ����ˮ�ܵ�����Ⱥ�ں�����ࡢ������ˮ��
(1)Ӳˮ������ �������֡�Ӳˮ�к��н϶�����Ըơ�þ�����Ӳˮ���������������������鷳�������п�������������ˮ��Ӳ�ȣ�
(2)Ӳˮ�ڼ��Ȼ����ʱ�������ˮ��[��Ҫ�ɷ���Mg��OH��2��CaCO3]�������п�������ϡ�����ȥ��ˮƿ���ϵ�ˮ����д���йط�Ӧ�Ļ�ѧ����ʽ�� ���� ����
������ˮ���������У����� ������ȥˮ�в��������ʣ�ͬʱ�������������ɱ����ClO2��һ�����͵�����ˮ��������������Ԫ������Ԫ�ص�������Ϊ�� ���ȵ�ԭ�ӽṹʾ��ͼΪ ��
(1)Ӳˮ������ �������֡�Ӳˮ�к��н϶�����Ըơ�þ�����Ӳˮ���������������������鷳�������п�������������ˮ��Ӳ�ȣ�
(2)Ӳˮ�ڼ��Ȼ����ʱ�������ˮ��[��Ҫ�ɷ���Mg��OH��2��CaCO3]�������п�������ϡ�����ȥ��ˮƿ���ϵ�ˮ����д���йط�Ӧ�Ļ�ѧ����ʽ�� ���� ����
������ˮ���������У����� ������ȥˮ�в��������ʣ�ͬʱ�������������ɱ����ClO2��һ�����͵�����ˮ��������������Ԫ������Ԫ�ص�������Ϊ�� ���ȵ�ԭ�ӽṹʾ��ͼΪ ��
(1)������ˮ������������У���1�֣�
(2) Mg��OH��2+2HCl=MgCl2+2H2O����CaCO3+2HCl=CaCl2+H2O+CO2��������2�֣���
�ǹ��ˣ� 71��64 ��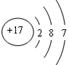 ����2�֣�
����2�֣�
(2) Mg��OH��2+2HCl=MgCl2+2H2O����CaCO3+2HCl=CaCl2+H2O+CO2��������2�֣���
�ǹ��ˣ� 71��64 ��
 ����2�֣�
����2�֣���1��Ӳˮ�е�Ca2����Mg2���ܺͷ���ˮ��Ӧ���������Գ������ݴ˿�������Ӳˮ���ڼ��ȵ������£�Ӳˮ�е�Ca2����Mg2�����γ�̼��ƺ�������þ�������ݴ˿��Խ���Ӳˮ��Ӳ�ȡ�
��2��̼��ƺ�������þ���ܺ����ᷴӦ������ʽ�ֱ���Mg��OH��2+2HCl=MgCl2+2H2O����CaCO3+2HCl=CaCl2+H2O+CO2����
��3��ˮ�в��������ʿ���ͨ�����˳�ȥ�����ݻ�ѧʽClO2��֪��Ԫ������Ԫ�ص�������Ϊ35.5�U32��71�U64����ԭ���ǵ���������17������ԭ�ӽṹʾ��ͼΪ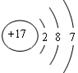 ��
��
��2��̼��ƺ�������þ���ܺ����ᷴӦ������ʽ�ֱ���Mg��OH��2+2HCl=MgCl2+2H2O����CaCO3+2HCl=CaCl2+H2O+CO2����
��3��ˮ�в��������ʿ���ͨ�����˳�ȥ�����ݻ�ѧʽClO2��֪��Ԫ������Ԫ�ص�������Ϊ35.5�U32��71�U64����ԭ���ǵ���������17������ԭ�ӽṹʾ��ͼΪ
 ��
��
��ϰ��ϵ�д�
�����Ŀ