��Ŀ����
Se�ǰ뵼����ϣ�Ҳ�Dz�������Ʒ�е�Ԫ�أ���ҵ����ȡ���ķ���֮һ����H2SO4��NaNO3������Se�Ĺ�ҵ���϶��õ�������(H2SeO3)����������(H2SeO4)Se�ǰ뵼����ϣ�Ҳ�Dz�������Ʒ�е�Ԫ�أ���ҵ����ȡ���ķ���֮һ����H2SO4��NaNO3������Se�Ĺ�ҵ���϶��õ�������(H2SeO3)����������(H2SeO4)�����������Ṳ�ȿ����������ᣬ��ӦΪH2SeO4+2HCl��H2SeO3+Cl2��+H2O����SO2ͨ����������Һ���е������������ݴˣ�����˵������ȷ����
- A.H2SeO4������ǿ��Cl2
- B.H2SeO3������ǿ��ϡH2SO4
- C.SeO2�Ļ�ԭ��ǿ��SO2
- D.����1mol Se��H2SeO3��SO2��H2O��1mol
A
������Ŀ�����������������ԭ��Ӧ���й�֪ʶ����֪�����ΪA��⣺������Ŀ�����������������ԭ��Ӧ���й�֪ʶ����֪�����ΪA�
������Ŀ�����������������ԭ��Ӧ���й�֪ʶ����֪�����ΪA��⣺������Ŀ�����������������ԭ��Ӧ���й�֪ʶ����֪�����ΪA�

��ϰ��ϵ�д�
�����Ŀ
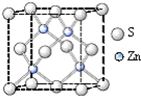
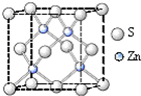 Ga��As��һ�������¿��Ժϳ�GaAs��GaAs��һ�����ͻ�����뵼����ϣ������ܱȹ����Խ����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȣ�
Ga��As��һ�������¿��Ժϳ�GaAs��GaAs��һ�����ͻ�����뵼����ϣ������ܱȹ����Խ����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȣ�