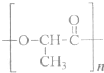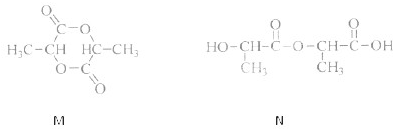��Ŀ����
��ѡ���⣩��19�֣�ͼ������ģ�ͷ��dz��õĿ�ѧ�о�������
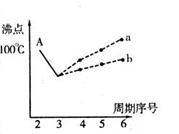
I����ͼ���о�����Ԫ�ص��⻯��ķе�仯���ɵ�ͼ��ͬͬѧ��ij����Ԫ���⻯��ķе�ı仯���ƻ������������ߡ�������a������b������A���Ӧ�ķе���100�棩������Ϊ��ȷ���� �������� ��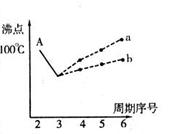
II��������ʹ�ý�������ʷ�����У�������ͭ��������֮�����ֽ����㷺Ӧ�õĽ�����ѧ��Ԥ��Ϊ���ѣ�Ti�����ѱ���Ϊ��δ�����͵Ľ��������Իش��������⣺
��1��22TiԪ�ػ�̬ԭ�ӵļ۵��Ӳ��Ų�ʽΪ ��
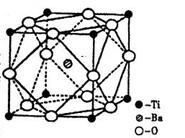
��2����Ti�Ļ������У����Գ���+2��+3��+4���ֻ��ϼۣ�������+4�۵�Ti��Ϊ�ȶ���ƫ���ᱵ�����ȶ��Ժã��۵糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�á�ƫ���ᱵ�����о����Ľṹʾ��ͼ����ͼ�������Ļ�ѧʽ�� ��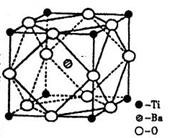
III��������60�껯����һ��ϡ�����廯����Xe[PtF6]���ϳɳ��������ˡ��������ԡ��Ĺ�������ļ����ڣ���ѧ������̺����믵ķ����������ȡ�
��1������Pt�ڲ�ԭ�ӵĶѻ���ʽ��ͭ���ɱ��е�CO2��ͬ����ͼ��������Pt������ʾ��ͼ����˵��Ptԭ���ھ����е�λ�� ��
��2��ϡ�����壨뱳��⣩�У�ֻ�н��ص���ܺϳɳ����ֻ����
�����ԭ���� ������ĸ���ţ�
| A��믵ĺ����ȽϷḻ | B��믵����ԭ�������� |
| C���ԭ�Ӱ뾶������С | D���ԭ�Ӱ뾶С���縺�Դ� |
��b��2�֣�
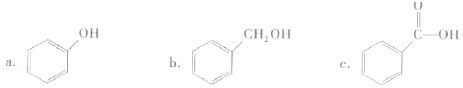
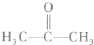
A����ʾ���⻯����ˮ����е����������ˮ���Ӽ���ڶ���������ϴ���������ǿ��ԶԶ���ڷ��Ӽ�����������������Ԫ���������⻯��ķе㲻�����ˮ����4�֣�
��1��3d24s2��2�֣� ��2��BaTiO3��3�֣�
��1��������İ˸�������������ģ�4�֣�
��2��C��2�֣�
��3�����ԣ�2�֣�
����

(A)�����ʽṹ�����ʡ�
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����ijһ�ֻ�ѧԪ�ء�

(1)T3+�ĺ�������Ų�ʽ��____________��
(2)Q��R��M�ĵ�һ�������ɴ�С��˳����___________________(��Ԫ�ط��ű�ʾ)��
(3)�����й�����Ԫ�ص�˵���У���ȷ����______________________(�����)��
��G���ʵ��۵����J���ʣ�����ΪG���ʵĽ�������ǿ
��J��X���ã�����J��������Һ���û���X
�۽�J
��RE3�е����QE4����Ҫ����Ϊǰ����Է��������ϴ�
��һ��Q2E4�����к�������Ҽ���һ���м�
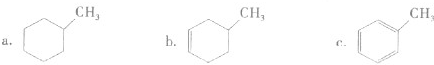
(4)���ô�����̨��̫�շ�����EQ9R����֪����������ԭ�Ӿ��γ�8���ӻ�2�����ȶ��ṹ����ֱ���η��ӣ���������λ����д����ṹʽ��_________________��
(5)G��R����ֱ�ӻ�������һ�����ӻ�����G3R���þ����������ʯī�IJ�״�ṹ��ÿ���У�Gԭ�ӹ���ƽ�������Σ�ÿ�������ε�������һ��Rԭ�ӡ������֮�仹����һ��������ԭ�ӡ�������Щ���ӵ�ԭ��Ӧ����____________(��G��R��Ԫ�ط���)��

(B)��ʵ�黯ѧ��
ij������ʾ����ʹ˫��ˮ�ֽ�Ĵ����кܶ��֣��������(������)�������ʹ���(��FeCl3)�������(��MnO2)�ȶ��ǽϺõĴ�����ijʵ��С��ͨ���ⶨ˫��ˮ�ֽ������O2��ѹǿ��̽���ֽ�����������Ѵ����Լ�̽����Ѵ������ʵĴ�������
(һ)̽��һ��
ʵ�鲽��
(1)����ƿ�м���50 mL 1.5����˫��ˮ
(2)�ֱ�����ƿ�м�
(3)�ɼ��ͼ�¼���ݡ�
(4)�������ݵó��±�
��ͬ������ѹǿ��ʱ��б�ʡ��ıȽ�
���� | ���� | ������ | �Ȼ�ͭ | �Ȼ��� | ����ͭ | �������� |
ѹǿ��ʱ���б�� | 0.191 87 | 0.002 42 | 0.007 93 | 0.030 5 | 0.015 47 | 1.833 6 |
�ٸá�̽��һ��ʵ���������_____________________________________________________��
�ڸ�ʵ�����ó��Ľ�����_______________________________________________________��
(��)̽�������������̴�����Ѵ�����
��ʵ��С���ͬѧ�ڽ���̽������ʵ��ʱ���õ���һϵ�е�ͼ�������ݡ��ο���ͼ�ͱ���ֱ�ش�������⡣

3%��˫��ˮ�벻ͬ�����������̵�ѹ����ʱ��ͼ
������ͬŨ�ȵ�˫��ˮ�ڲ�ͬ�����Ķ��������������ռ���ͬ״����ͬ���O2����ʱ��
MnO2 ʱ�� H2O2 | |||
1.5�� | 223 s | 67 s | 56 s |
3.0�� | 308 s | 109 s | 98 s |
4.5�� | 395 s | 149 s | 116 s |
����ͼ�������������ǿ��Եó���
��ͬŨ�ȵ�˫��ˮ�ķֽ��������Ŷ����������������Ӷ�_________________�������Ӧʱ��_______________��
�������ʵ�����ͽ�ʡҩƷ�ĽǶ��ۺϷ���������Ϊ������ѡ��3.0%��˫��ˮ������___________ g�Ķ���������ʹʵ��Ч����ѡ����жϵ�������______________________��
�ݸ�С���ijͬѧͨ���������ݵó��˵�����������ͬʱ˫��ˮ��Ũ��ԽС��Ӧ����Խ��Ľ��ۣ�����Ϊ�Ƿ���ȷ____________�����������________________________________��