��Ŀ����
��15�֣��ף��ң���������������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ʵ�߱�ʾ���ۼ���X��Y��ͬ�ɲ�ͬ��

��֪���ף��Ҿ���������ͬ�����ʼ������ҷ����û���Ӧ�������������������Ӿ����е������ܵ����������б�������ͬһ�ྦྷ���еķ��ӣ����ڳ����³�Һ̬���ܲ�������10���ӵ����ӣ�������������������ϡ��ȵ���ԭ������������ͬ��������ԭ�����ķ��ӻ����ӻ���Ϊ�ȵ����壩��
��1��д��Һ̬��������ֵȵ������ӵĵ��뷽��ʽ�� ��
��2��д�������ҷ����û���Ӧ�ķ�Ӧ����ʽ�� ��
��3������Ŀǰ��Ҫ����Դ֮һ��
�� �������ڴ������������µõ���ȼ�Ե��������壬�䷴Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�� �ִ����ܵ���У����ñ���ȼ�ϵ�ص�ԭ�ϣ��ڼ��Խ��ʣ�KOH��Һ��������£��为����Ӧ�ĵ缫����ʽΪ ��
��4����������������Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ����

��֪���ף��Ҿ���������ͬ�����ʼ������ҷ����û���Ӧ�������������������Ӿ����е������ܵ����������б�������ͬһ�ྦྷ���еķ��ӣ����ڳ����³�Һ̬���ܲ�������10���ӵ����ӣ�������������������ϡ��ȵ���ԭ������������ͬ��������ԭ�����ķ��ӻ����ӻ���Ϊ�ȵ����壩��
��1��д��Һ̬��������ֵȵ������ӵĵ��뷽��ʽ�� ��
��2��д�������ҷ����û���Ӧ�ķ�Ӧ����ʽ�� ��
��3������Ŀǰ��Ҫ����Դ֮һ��
�� �������ڴ������������µõ���ȼ�Ե��������壬�䷴Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�� �ִ����ܵ���У����ñ���ȼ�ϵ�ص�ԭ�ϣ��ڼ��Խ��ʣ�KOH��Һ��������£��为����Ӧ�ĵ缫����ʽΪ ��
��4����������������Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ����
��1��2H2O
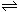 H3O++OH��
H3O++OH�� ��2�� SiO2+2C
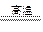 Si+2CO��
Si+2CO����3�� ��CH4+H2O
 CO+3H2 ��C H 4 + 10 OH- - 8 e- = CO32- + 7 H2O��
CO+3H2 ��C H 4 + 10 OH- - 8 e- = CO32- + 7 H2O����4�� NH4+ +H2O
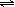 NH3��H2O + H+
NH3��H2O + H+��

��ϰ��ϵ�д�
 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
�����Ŀ
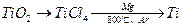
 2Cl2(g) = TiCl4(s)+O2(g)����H=+141kJ/mol
2Cl2(g) = TiCl4(s)+O2(g)����H=+141kJ/mol ��Ĺ��ۼ����ڵ������۷е�һ���ܸ�
��Ĺ��ۼ����ڵ������۷е�һ���ܸ�