��Ŀ����
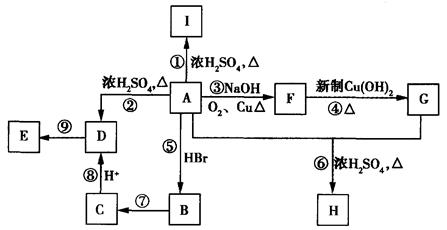
(18��)A��B��һ�������¿ɺϳɷ����廯����E��G�ڱ�״���������壬������µ��ܶ�Ϊ1.25g/L�������ʼ��ת����ϵ������ʾ��
��ش��������⣺
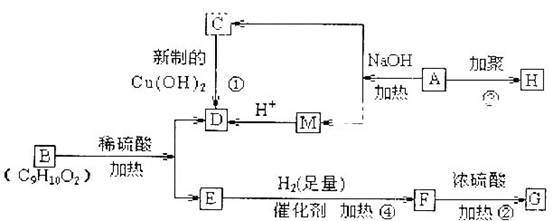
(1)G�еĹ������� (�û�ѧʽ��ʾ)��D�еĹ����������� ��F�Ľṹ��ʽΪ ��
(2)ָ����Ӧ���ͣ��� ���� ���� ��
(3) д����ѧ����ʽ��A��C ��
C�����Ƶ�������ͭ����Һ��Ӧ�� ��
(4)��������������B��ͬ���칹���� �֡�
����FeCl3��Һ����ɫ ���ܷ���������Ӧ �۱�����ֻ������ȡ����

��ش��������⣺
(1)G�еĹ������� (�û�ѧʽ��ʾ)��D�еĹ����������� ��F�Ľṹ��ʽΪ ��
(2)ָ����Ӧ���ͣ��� ���� ���� ��
(3) д����ѧ����ʽ��A��C ��
C�����Ƶ�������ͭ����Һ��Ӧ�� ��
(4)��������������B��ͬ���칹���� �֡�
����FeCl3��Һ����ɫ ���ܷ���������Ӧ �۱�����ֻ������ȡ����
(ÿ��2�֣���18��)
��1��
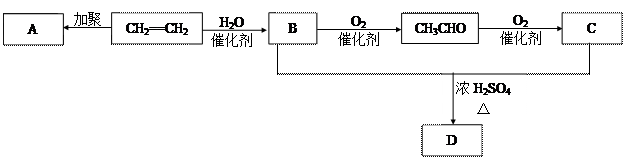
(2)��ȡ����Ӧ (������Ӧ)�� ��ȥ��Ӧ�ۼӾ۷�Ӧ
(3)2CH3CH2OH+O2 2CH3CHO+H2O
2CH3CHO+H2O
CH3CHO+2Cu(OH)2 CH3COOH+Cu2 O��+2H2O
CH3COOH+Cu2 O��+2H2O
(4) 3
��1��

(2)��ȡ����Ӧ (������Ӧ)�� ��ȥ��Ӧ�ۼӾ۷�Ӧ
(3)2CH3CH2OH+O2
 2CH3CHO+H2O
2CH3CHO+H2OCH3CHO+2Cu(OH)2
 CH3COOH+Cu2 O��+2H2O
CH3COOH+Cu2 O��+2H2O(4) 3
��

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
�����Ŀ
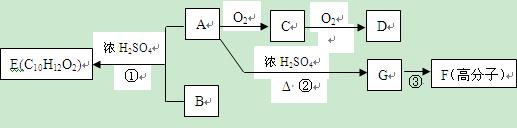
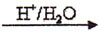 RCOOH����ѧ���������Ⱥ�����ѡ����ǿ��ҩЧ�ȶ���������ȫ�Ķ�̱����Ӳݳ��ݼ������ĺϳ�·�����£�
RCOOH����ѧ���������Ⱥ�����ѡ����ǿ��ҩЧ�ȶ���������ȫ�Ķ�̱����Ӳݳ��ݼ������ĺϳ�·�����£�
 ��̼ԭ�ӡ�
��̼ԭ�ӡ� ��
�� OH ��ȫ��Ӧ��
OH ��ȫ��Ӧ��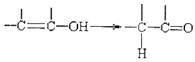
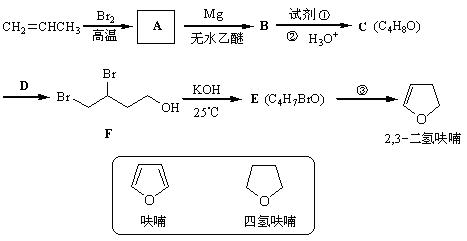
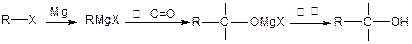
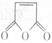 �Ǻϳ�ijЩҩ����м��塣����ƺ��������ɻ�����
�Ǻϳ�ijЩҩ����м��塣����ƺ��������ɻ�����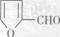 �ϳ�
�ϳ�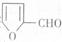 ��400���϶Ƚ����������������ܷ������ʻ���Ӧ���ۺϳɷ�Ӧ����ͼ��ʾ����ʾ�����£�
��400���϶Ƚ����������������ܷ������ʻ���Ӧ���ۺϳɷ�Ӧ����ͼ��ʾ����ʾ�����£�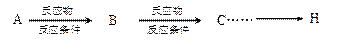
 ��C��ʹ��ˮ��ɫ��1Ħ��C��2Ħ��������ȫ�ӳɣ������������ÿ��̼ԭ���϶�������ԭ�Ӻ���ԭ�ӡ�A��C��B��D�ֱ������ͬ��ͨʽ��A�ڴ�����������������Ӧ�ɵõ�B����ͬ��ͬѹ��B�뵪�����ܶ���ͬ��Dû��ͬ����칹�壬��������̬�������Ʒֱ���:
��C��ʹ��ˮ��ɫ��1Ħ��C��2Ħ��������ȫ�ӳɣ������������ÿ��̼ԭ���϶�������ԭ�Ӻ���ԭ�ӡ�A��C��B��D�ֱ������ͬ��ͨʽ��A�ڴ�����������������Ӧ�ɵõ�B����ͬ��ͬѹ��B�뵪�����ܶ���ͬ��Dû��ͬ����칹�壬��������̬�������Ʒֱ���: RX+H2O��EΪ�߷��ӻ��������ʽΪ((C9H8O2)n��H����ʽΪC18H15O6Na��I�к���һ��������������һ����Ԫ�ӻ���
RX+H2O��EΪ�߷��ӻ��������ʽΪ((C9H8O2)n��H����ʽΪC18H15O6Na��I�к���һ��������������һ����Ԫ�ӻ���