��Ŀ����
�мס��ҡ�����֧�Թܣ��ֱ�����������ʺ۲���֧�Թܵ���ɫ��������ɫ��dz����
�ף�10 mL 0.01 mol��L��1��FeCl3��Һ��10 mL 0.01 mol��L��1��KSCN��Һ
�ң�5 mLˮ��10 mL 0.01 mol��L��1��FeCl3��Һ5 mL 0.01 mol��L��1��KSCN��Һ
����10 mL 0.1 mol��L�� 1��FeCl3����Һ��10 mL 0.1 mol��L��1��KSCN��Һ
1��FeCl3����Һ��10 mL 0.1 mol��L��1��KSCN��Һ
A�����Թ� B�����Թ� C�����Թ� D�����ж�
��ϰ��ϵ�д�
�����Ŀ
ijѧ���� Na2CO3�� KHCO3 ��ɵ�ij��������ʵ�飬�����������(��������ʵ���Ũ������Ҳ�����HCl�Ļӷ�)
ʵ����� | �� | �� | �� | �� |
�������/mL |
| 50 | 50 | 50 |
��������/g | 3.06 | 6.12 | 9.18 | 12.24 |
�����������/L(���) | 0.672 | 1.344[ | 1.568 | 1.344 |
�����йص�˵���У���ȷ����
A����������ʵ���Ũ��Ϊ 2 mol��L��1
B��ԭ�������Ʒ�� n(Na2CO3)��n(KHCO3)��1:1
C��ʵ����У���������
D��ʵ��ܷ�Ӧ��������������� 40ml �ĸ�������Һ���ܰ�12.24g �Ļ����ȫ����Ӧ


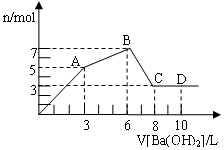
 50
50 CO2(g) + 3H2(g)
CO2(g) + 3H2(g)  H(298K)��+ 49.4 kJ/mol��ʵ���ã��ﵽƽ��״̬ʱ����������19.76 kJ����
H(298K)��+ 49.4 kJ/mol��ʵ���ã��ﵽƽ��״̬ʱ����������19.76 kJ���� ��ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
��ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
 C6H5COOH
C6H5COOH