��Ŀ����
��11�֣��о�ȼ�ϵ�ȼ�պͶ���Ⱦ���������������������ڷ�ֹ������Ⱦ����Ҫ���塣
��1����úת��Ϊ�������ȼ�ϣ�
��֪��H2(g)+1/2O2(g)=H2O(g)  H= ?241��8kJ/mol
H= ?241��8kJ/mol
C(s)+1/2O2(g)=CO(g)  H= ?110��5kJ/mol
H= ?110��5kJ/mol
д����̿��ˮ������Ӧ��H2��CO���Ȼ�ѧ����ʽ ��
��2��һ�������£����ܱ������ڣ�SO2��������SO3���Ȼ�ѧ����ʽΪ��2SO2(g)+O2(g) 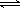 2SO3(g)��
2SO3(g)��
��H=?a kJ/mo1������ͬ������Ҫ��õ�2akJ��������������ʵ����ʵ���������
A��4mo1 SO2��2mol O2���������� B��4mol SO2��2mo1 O2��2mol SO3
C��4mol SO2��4mo1 O2������ D��6mo1 SO2��4mo1 O2
��3������β����NOx��CO�����ɼ�ת����
����֪����������NO�ķ�ӦΪ��N2(g)+O2(g)  2NO(g)
2NO(g)  H��0
H��0
��һ���¶��µĶ����ܱ������У���˵���˷�Ӧ�Ѵ�ƽ�����
A��ѹǿ���� B���������ƽ����Է�����������
C��2v��(N2)��v��(NO) D�� N2������������ٸı�
������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO(g)=2C(s)+O2(g)  H��0��
H��0��
�����������ܷ�ʵ�ֵ����� ��
��4��ȼ��CO��H2��һ�������¿����ת����CO(g)��H2O(g)  CO2(g)��H2(g)����420��ʱ��ƽ�ⳣ��K=9������Ӧ��ʼʱ��CO��H2O��Ũ�Ⱦ�Ϊ0��1mol/L����CO�ڴ˷�Ӧ�����µ�ת����Ϊ ��
CO2(g)��H2(g)����420��ʱ��ƽ�ⳣ��K=9������Ӧ��ʼʱ��CO��H2O��Ũ�Ⱦ�Ϊ0��1mol/L����CO�ڴ˷�Ӧ�����µ�ת����Ϊ ��
��1��C(s)+H2O(g)=CO(g)+H2(g)  H=+131��3kJ/mol (3��)(����ʽ���±���ȷ�ɸ�1��)
H=+131��3kJ/mol (3��)(����ʽ���±���ȷ�ɸ�1��)
��2��D (2��)
��3����CD (2��)
�ڸ÷�Ӧ���������ؼ��ķ�Ӧ������ G=
G= H��T
H��T S,
S,  G��0,����ʵ�֡� (2��)
G��0,����ʵ�֡� (2��)
��4��75% (2��)
���������������1����֪��H2(g)+1/2O2(g)=H2O(g)  H1= ?241��8kJ/mol ��C(s)+1/2O2(g)="CO(g)"
H1= ?241��8kJ/mol ��C(s)+1/2O2(g)="CO(g)"  H2= ?110��5kJ/mol���ݸ�˹���ɣ��÷���ʽ2��ȥ����ʽ1���ɵã�д����̿��ˮ������Ӧ��H2��CO���Ȼ�ѧ����ʽ��1��C(s)+H2O(g)=CO(g)+H2(g)
H2= ?110��5kJ/mol���ݸ�˹���ɣ��÷���ʽ2��ȥ����ʽ1���ɵã�д����̿��ˮ������Ӧ��H2��CO���Ȼ�ѧ����ʽ��1��C(s)+H2O(g)=CO(g)+H2(g)  H=+131��3kJ/mol ����2���ɷ�Ӧ����ʽ��֪����2mol��SO3ʱ����a kJ����õ�2a kJ��������������4molSO3�����ڷ�Ӧ�ǿ��淴Ӧ����������ȫ���е��ף�����Ҫ�õ�4molSO3��SO2��O2���ʵ�������Ҫ����4mol�� 2mol����D���ϣ�Bѡ���м�������Ӧ�����淴Ӧ�����շų�������С��2akJ����ѡD����3����A�����ڷ�Ӧǰ��������䣬��ѹǿʼ�ղ��䲻����Ϊƽ���ж����ݣ����� B�����ڷ�Ӧǰ���������������ʵ��������ı䣬�ʻ������ƽ����Է����������䣬�ʲ�����Ϊƽ���ж����ݣ�����ѡCD�����ɷ�Ӧ2CO(g)=2C(s)+O2(g)
H=+131��3kJ/mol ����2���ɷ�Ӧ����ʽ��֪����2mol��SO3ʱ����a kJ����õ�2a kJ��������������4molSO3�����ڷ�Ӧ�ǿ��淴Ӧ����������ȫ���е��ף�����Ҫ�õ�4molSO3��SO2��O2���ʵ�������Ҫ����4mol�� 2mol����D���ϣ�Bѡ���м�������Ӧ�����淴Ӧ�����շų�������С��2akJ����ѡD����3����A�����ڷ�Ӧǰ��������䣬��ѹǿʼ�ղ��䲻����Ϊƽ���ж����ݣ����� B�����ڷ�Ӧǰ���������������ʵ��������ı䣬�ʻ������ƽ����Է����������䣬�ʲ�����Ϊƽ���ж����ݣ�����ѡCD�����ɷ�Ӧ2CO(g)=2C(s)+O2(g)  H��0����֪�÷�Ӧ���������ؼ��ķ�Ӧ������
H��0����֪�÷�Ӧ���������ؼ��ķ�Ӧ������ G=
G= H��T
H��T S,
S,  G��0,����ʵ�֡�
G��0,����ʵ�֡�
��4���⣺��ƽ��ʱCO��Ũ�ȱ仯��Ϊxmol/L����
CO��g��+H2O��g��?CO2��g��+H2��g��
��ʼ��mol/L����0��1 0��1 0 0
�仯��mol/L����c c c c
ƽ�⣨mol/L����0��1-c 0�� 1-c c c
��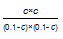 =9 ���c=0��075����һ����̼�ڴ������µ�ƽ��ת����=75%���ʴ�Ϊ��75%��
=9 ���c=0��075����һ����̼�ڴ������µ�ƽ��ת����=75%���ʴ�Ϊ��75%��
���㣺���⿼���˻�ѧƽ����йؼ��㡢��ѧƽ�ⳣ����Ӧ�ã��жϷ�Ӧ���еij̶ȡ��жϷ�Ӧ���еķ���ȡ�

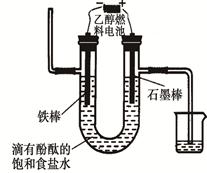
��Ԫ������Ҫ�Ľ���Ԫ�أ��������ڹ�ҵ��������ʹ�õ���Ϊ�㷺�������кܶ���Ҫ�Ļ����P�仯ѧ��Ӧ��������ˮ��Ӧ��3Fe(s)��4H2O(g)��Fe3O4(s)��4H2(g) ��H
(1)������Ӧ��ƽ�ⳣ������ʽK��_______��
(2) ��֪����3Fe(s)��2O2(g)��Fe3O4(s) ��H1����1118.4kJ/mol
��2H2(g)��O2(g)��2H2O(g) ��H2����483.8kJ/mol
��2H2(g)��O2(g)��2H2O(l) ��H3����571.8kJ/mol
���H��_______��
(3)��t0Cʱ,�÷�Ӧ��ƽ�ⳣ��K��16����2L���º����ܱ�����������,�ֱ��±���ʾ�������ʣ���Ӧ����һ��ʱ���ﵽƽ�⡣
| | Fe | H2O��g�� | Fe3O4 | H2 |
| ��/mol | 1.0 | 1.0 | 1.0 | 1.0 |
| ��/mol | 1.0 | 1.5 | 1.0 | 1.0 |
�ټ�������H2O��ƽ��ת����Ϊ_______ (�������һλС��)��
������˵����ȷ����_______ (����)
A.������ѹǿ�㶨����Ӧ�ﵽƽ��״̬
B.�������������ܶȺ㶨����Ӧ�ﵽƽ��״̬
C.��������H2O��ƽ��ת���ʴ�����������H2O��ƽ��ת����
D.����Fe3O4�������H2O��ת����
(4)����(3)��װ�ø�Ϊ���ݾ���(������罻������)װ�ã����±�������ʼ���ʣ���ʼʱ��ƽ���ĸ����ʵ���������
| | Fe | H2O��g�� | Fe3O4 | H2 |
| ��ʼ/mol | 3.0 | 4.0 | 0 | 0 |
| ƽ��/mol | m | n | p | q |
���ڴ�ƽ����װ���м�������A��B��C����״���µĸ����ʣ�������
| | Fe | H2O��g�� | Fe3O4 | H2 |
| A/mol | 3.0 | 4.0 | 0 | 0 |
| B/mol | 0 | 0 | 1 | 4 |
| C/mol | m | n | p | q |
���������淴Ӧ��һ�δﵽƽ��״̬��,������װ����H2�İٷֺ������ɴ�С��˳�����еĹ�ϵ��
________(��A��B��C��ʾ����
(5)��֪Fe(OH)3��Ksp��2.79��10��39����FeCl3��Һ������ʾ��ǿ�����ԣ���ijFeCl3��Һ��pHΪ3,�����Һ��c(Fe3+)��________mol ? L-1 (�������3λ��Ч����)
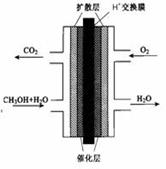
 2PbSO4��2H2O����ش��������⣨�������⡢����������ԭ����
2PbSO4��2H2O����ش��������⣨�������⡢����������ԭ���� 
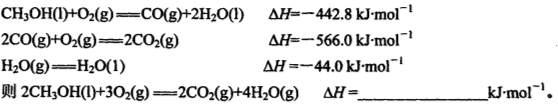
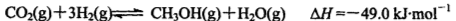
 mol;
mol;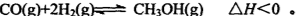

 ���>������<����=����
���>������<����=����
 2SO3��g�� ��H =" a" kJ·mol��1����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99 kJ����ش�
2SO3��g�� ��H =" a" kJ·mol��1����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99 kJ����ش� 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol  2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��
2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��

 ������ã�ʹ��Ӧ������������ɣ�
������ã�ʹ��Ӧ������������ɣ� 2NH3(g) ��H����92 kJ/mol
2NH3(g) ��H����92 kJ/mol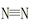 ����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��