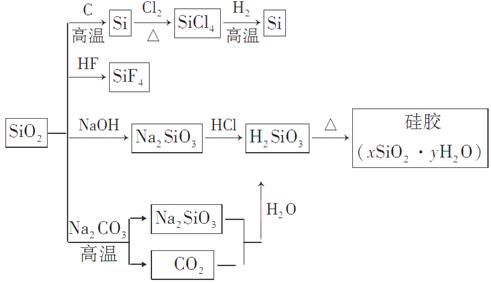
��Ŀ����
�輰�仯����������ִ������ķ�չ��������ס���ش������й����⣺
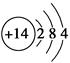
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӡ���ʯӢ���ά�����մ�����
����ͨ�������ݹ�̫���ܵ��
(3)�����£�SiCl4ΪҺ̬���е�Ϊ57.6 �棬�ڿ�����ð�������Ʊ��ߴ��ȹ���м����SiCl4������Һ̬���ʣ���Ҫ�õ��ߴ���SiCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��________________________________
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӡ���ʯӢ���ά�����մ�����
����ͨ�������ݹ�̫���ܵ��
| A���٢ڢ� | B���ۢܢ� | C���ڢۢ� | D���٢ۢ� |
(1)
(2)D
(3)��������������SiCl4��3H2O=H2SiO3��4HCl��HCl��ˮ������������

(2)D
(3)��������������SiCl4��3H2O=H2SiO3��4HCl��HCl��ˮ������������
(1)��ԭ�ӽṹʾ��ͼΪ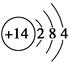 ��(2)������Ͽ��ӵ�ˮ���к��й����Σ�ʯӢ���ά����Ҫ�ɷ���SiO2�����ǹ����Σ��մ������к��й����Σ������к��й����Σ���̫���ܵ���к��е��ʹ�ijɷ֣����������Ρ�(3)��SiCl4�ķе�͵����ʿ���ѡ������ķ�������������ΪSiCl4�ܹ�ˮ�⣬���Ա���ѡ����ˮ(��ˮ����)�Ļ������иò�����SiCl4�ڿ�������ˮ������Ӧ����H2SiO3��HCl������HCl�ڿ���������ˮ��������������
��(2)������Ͽ��ӵ�ˮ���к��й����Σ�ʯӢ���ά����Ҫ�ɷ���SiO2�����ǹ����Σ��մ������к��й����Σ������к��й����Σ���̫���ܵ���к��е��ʹ�ijɷ֣����������Ρ�(3)��SiCl4�ķе�͵����ʿ���ѡ������ķ�������������ΪSiCl4�ܹ�ˮ�⣬���Ա���ѡ����ˮ(��ˮ����)�Ļ������иò�����SiCl4�ڿ�������ˮ������Ӧ����H2SiO3��HCl������HCl�ڿ���������ˮ��������������
 ��(2)������Ͽ��ӵ�ˮ���к��й����Σ�ʯӢ���ά����Ҫ�ɷ���SiO2�����ǹ����Σ��մ������к��й����Σ������к��й����Σ���̫���ܵ���к��е��ʹ�ijɷ֣����������Ρ�(3)��SiCl4�ķе�͵����ʿ���ѡ������ķ�������������ΪSiCl4�ܹ�ˮ�⣬���Ա���ѡ����ˮ(��ˮ����)�Ļ������иò�����SiCl4�ڿ�������ˮ������Ӧ����H2SiO3��HCl������HCl�ڿ���������ˮ��������������
��(2)������Ͽ��ӵ�ˮ���к��й����Σ�ʯӢ���ά����Ҫ�ɷ���SiO2�����ǹ����Σ��մ������к��й����Σ������к��й����Σ���̫���ܵ���к��е��ʹ�ijɷ֣����������Ρ�(3)��SiCl4�ķе�͵����ʿ���ѡ������ķ�������������ΪSiCl4�ܹ�ˮ�⣬���Ա���ѡ����ˮ(��ˮ����)�Ļ������иò�����SiCl4�ڿ�������ˮ������Ӧ����H2SiO3��HCl������HCl�ڿ���������ˮ��������������
��ϰ��ϵ�д�
�����Ŀ