��Ŀ����
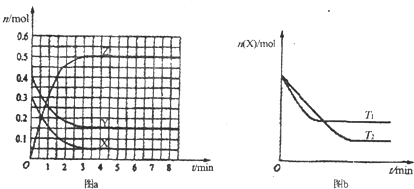
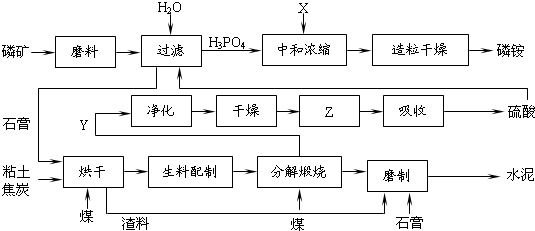
��2010?���ն�ģ���ҹ�����Դ��ȱ��ɽ��ij���������ۺ�����������ʣ��ʯ�����������ļ����ѹأ����ҽ���李������ˮ��ʵʩ������������Ҫ��������ͼ��ʾ����֪��������������ᡢ��ǿ�ᣩ��
�Ը�������������գ�
��1��д��X�������к����������淋����ӷ���ʽ��
��2��ʯ�ྭ��ˮ���뽹̿��������������Y����ʯ�ҡ�������̼��д���÷�Ӧ�Ļ�ѧ����ʽ��
��3������������ˮ��Ĺ����м���ú�������ǣ�

�Ը�������������գ�
��1��д��X�������к����������淋����ӷ���ʽ��
2NH3+H3PO4=2NH4++HPO42-
2NH3+H3PO4=2NH4++HPO42-
����2��ʯ�ྭ��ˮ���뽹̿��������������Y����ʯ�ҡ�������̼��д���÷�Ӧ�Ļ�ѧ����ʽ��
2CaSO4+C
2SO2��+CO2��+2CaO
| ||
2CaSO4+C
2SO2��+CO2��+2CaO
������Z���������Ϊ
| ||
��ת������Ӵ��ң�
��ת������Ӵ��ң�
����3������������ˮ��Ĺ����м���ú�������ǣ�
�ṩ����
�ṩ����
����������1������������ԣ���ϲ���Ϊ��刺�֪XΪNH3��
��2��ʯ�ྭ��ˮ���뽹̿��������������Y����ʯ�ҡ�������̼��˵������������ԭ��Ӧ����YӦΪ�������ɽ�һ��������Ϊ�����������������
��3������ú���ں�ɣ�Ӧ�ṩ���ܣ�
��2��ʯ�ྭ��ˮ���뽹̿��������������Y����ʯ�ҡ�������̼��˵������������ԭ��Ӧ����YӦΪ�������ɽ�һ��������Ϊ�����������������
��3������ú���ں�ɣ�Ӧ�ṩ���ܣ�
����⣺��1������������ԣ���ϲ���Ϊ��刺�֪XΪNH3����Ӧ�����ӷ���ʽΪ2NH3+H3PO4=2NH4++HPO42-��
�ʴ�Ϊ��2NH3+H3PO4=2NH4++HPO42-��
��2��ʯ�ྭ��ˮ���뽹̿��������������Y����ʯ�ҡ�������̼��˵������������ԭ��Ӧ����YӦΪ��������Ӧ�ķ���ʽΪ2CaSO4+C
2SO2��+CO2��+2CaO��SO2�ɽ�һ���ڽӴ����б�������Ϊ�����������������ᣬ
�ʴ�Ϊ��2CaSO4+C
2SO2��+CO2��+2CaO����ת������Ӵ��ң���
��3������ú���ں�ɣ�Ӧ�ṩ���ܣ��ʴ�Ϊ���ṩ���ܣ�
�ʴ�Ϊ��2NH3+H3PO4=2NH4++HPO42-��
��2��ʯ�ྭ��ˮ���뽹̿��������������Y����ʯ�ҡ�������̼��˵������������ԭ��Ӧ����YӦΪ��������Ӧ�ķ���ʽΪ2CaSO4+C
| ||
�ʴ�Ϊ��2CaSO4+C
| ||
��3������ú���ں�ɣ�Ӧ�ṩ���ܣ��ʴ�Ϊ���ṩ���ܣ�
�����������ۺϿ��������εĹ�ҵ������Ӧ�ã���Ŀ�ѶȲ������ʱע�������ʵ����ʺͲ����жϲμӷ�Ӧ�����ʣ�Ϊ������Ĺؼ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ