��Ŀ����
����Ŀ���о������к������Ҫ��SO2��H2S����ת��������Ҫ���塣
��1����ʪ�����£�д��������SO2ת��ΪHSO3-�ķ���ʽ��_____��
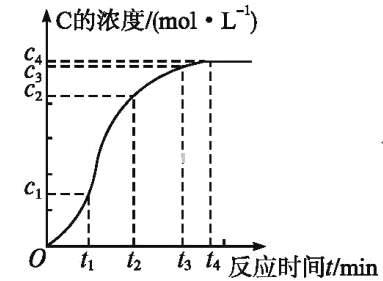
��2�������е�����ɽ�������H2S��������Ӧ������SO42-��������Ӧ�������仯ʾ��ͼ��ͼ��

1mol H2S��g��ȫ��������SO42-���Ȼ�ѧ����ʽΪ___________________________��
��3�������������������ӽ���Ĥȼ�ϵ�ؿ������ô�������SO2������������װ��ʾ��ͼ��ͼ��

�����ӵ���������Ϊ_______________________��������A��B��������B��A������
�ڸ����ĵ缫��ӦʽΪ_________________________________________________________________________________________��
��4��ȼú��������������Ǽ��ٴ����к�������Ⱦ�Ĺؼ���SO2�����ѳ���һ�ֹ�ҵ������ͼ��

���ô�����Һ����SO2������ת��ΪHSO3-����Ӧ�����ӷ���ʽ��_________________��
����ʯ�������������������Ż����ճأ����п���������SO2�����ʵĻ�ѧʽ��_____________________________��
���𰸡�SO2+H2O![]() H2SO3��H2SO3
H2SO3��H2SO3![]() H++HSO3-H2S(g)+2O2(g)=SO42-(aq)+2H+(aq) ��H=-803.39kJ/mol��A��BSO2-2e-+2H2O=SO42-+4H+H2O+2SO2+CO32-=2HSO3-+CO2NaOH��Ca(OH)2
H++HSO3-H2S(g)+2O2(g)=SO42-(aq)+2H+(aq) ��H=-803.39kJ/mol��A��BSO2-2e-+2H2O=SO42-+4H+H2O+2SO2+CO32-=2HSO3-+CO2NaOH��Ca(OH)2
��������
��1����������Ϊ������������ˮ���������ᣬ������Ϊ������ʣ����ֵ��������������������������ӣ����ӷ���ʽ��SO2+H2O![]() H2SO3��H2SO3
H2SO3��H2SO3![]() H++HSO3-��
H++HSO3-��
��2����ͼ��֪����һ���Ȼ�ѧ��ӦΪ��H2S��g��+0.5O2��g��=S��s��+H2O��g����H=-221.19 kJmol-1��
�ڶ�����ӦΪ��S��s��+1.5O2��g��+H2O��g��=2H+��aq��+SO42-��aq������H=-585.20 kJmol-1��
���ݸ�˹���ɣ���һ����ڶ�������ʽ��ӵã�H2S��g��+2O2��g��=SO42-��aq��+2H+��aq����H=-806.39 kJmol-1��
��3���ٶ���������������Ӧ������������ԭ��Ӧ�����Զ����������ڵ缫Ϊ�������������ڵ缫Ϊ������ԭ������������������������������ƶ�����Ϊ����A��B��
�ڶ��������ڸ���ʧȥ���ӷ���������Ӧ���缫��ӦʽΪ��SO2-2e-+2H2O=SO42-+4H+��
��4����̼������Һ��ͨ������Ķ�������Ӧ�������������ƺͶ�����̼�����ӷ���ʽ��H2O+2SO2+CO32-=2HSO3-+CO2��
���������У����������ƿ�����ʯ���鷴Ӧ����������Ƴ������������ơ���������Ϊ����������ܹ����Һ��Ӧ����NaOH������Ca(OH)2�����������ն���������

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�����Ŀ������ͼ,����������̽�����������ʡ�ʵ��ʱ��NaOH�����ϵμ���Ũ��ˮ,��������һ������������档�±��ж�ʵ�����������Ľ�����ȷ����
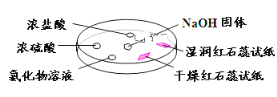
ѡ�� | ʵ������ | ���� |
A | �����ɫʯ����ֽ����ɫ,ʪ���ɫʯ����ֽ���� | NH3��һ�ֿ����Լ� |
B | Ũ���ḽ������������ | NH3��Ũ���������Ӧ |
C | �Ȼ�����Һ����� | ����Һһ����MgCl2��Һ |
D | Ũ���ḽ���������� | NH3��Ũ����ӷ�����HCl��Ӧ������NH4Cl���� |
A. A B. B C. C D. D