��Ŀ����
��һ������Na2O2��NaHCO3�Ļ�Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ���100mLϡ������ǡ����ȫ��Ӧ�����ɵ������������Ϊ2.24L����״���£����ٽ�������ͨ����һ�ݻ�����У���ַ�Ӧ�õ�O2 2.016L����״���£�����ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ϡ��������ʵ���Ũ���ǣ� ��| A | B | C | D | |
| Na2O2��NaHCO3�����ʵ���֮�� | 8��1 | 9��2 | 8��1 | 2��9 |
| ԭϡ��������ʵ�����Ũ�� | 1.4 | 1.4 | 1.8 | 1.3 |
A��A
B��B
C��C
D��D
���𰸡���������һ�ݷ�����Ӧ��2Na2O2+4HCl=4NaCl+2H2O+O2 ����NaHCO3+HCl=NaCl+H2O+CO2����������ΪO2��CO2��
����һ�����ɵĸ������壬ͨ��ڶ���Na2O2��NaHCO3�Ļ�Ϸ�ĩ��������Ӧ2Na2O2+2CO2=2Na2CO3+O2�����ݶ�����̼�Ƿ���������۵ķ������㣮
����⣺2.24L�����������ʵ���Ϊ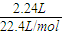 =0.1mol��
=0.1mol��
2.016LO2�����ʵ���Ϊ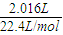 =0.09mol��
=0.09mol��
2Na2O2+2CO2=2Na2CO3+O2 ��n
2 2 1
n m 0.1mol-0.09mol=0.01mol
n=m=0.02mol��
1����CO2��ȫ��Ӧ�����һ������CO2��Ϊ0.02mol��O2Ϊ0.1mol-0.02mol=0.08mol
2 Na2O2+4 HCl=4NaCl+2H2O+O2
0.16mol 0.32mol 0.08mol
NaHCO3+HCl=NaCl+H2O+CO2
0.02mol 0.02mol 0.02mol
����Na2O2Ϊ0.16mol��NaHCO3Ϊ0.02mol���������Na2O2��NaHCO3�����ʵ���֮��0.16mol��0.02mol=8��1��
���ĵ�HClΪ0.32mol+0.02mol=0.34mol�����ʵ���Ũ��Ϊ =3.4mol/L��
=3.4mol/L��
2����CO2δ��ȫ��Ӧ����Na2O2Ӧ��0.02 mol��
2Na2O2+4HCl=4NaCl+2H2O+O2
0.02mol 0.04mol 0.01mol
���һ������O2��Ϊ0.01mol������CO2Ϊ0.1mol-0.01mol=0.09mol��
NaHCO3+HCl=NaCl+H2O+CO2
0.09mol 0.09mol 0.09mol
����Na2O2Ϊ0.02mol��NaHCO3Ϊ0.09mol���������Na2O2��NaHCO3�����ʵ���֮��0.02mol��0.09mol=2��9��
���ĵ�HClΪ 0.04mol+0.09mol=0.13mol�����ʵ���Ũ��Ϊ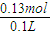 =1.3mol/L��
=1.3mol/L��
��ѡ��D��
���������⿼������ļ��㣬�ѶȽϴؼ��Ƕ�����̼��������Ƶķ�Ӧ���۽��м��㣮
����һ�����ɵĸ������壬ͨ��ڶ���Na2O2��NaHCO3�Ļ�Ϸ�ĩ��������Ӧ2Na2O2+2CO2=2Na2CO3+O2�����ݶ�����̼�Ƿ���������۵ķ������㣮
����⣺2.24L�����������ʵ���Ϊ
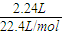 =0.1mol��
=0.1mol��2.016LO2�����ʵ���Ϊ
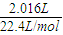 =0.09mol��
=0.09mol��2Na2O2+2CO2=2Na2CO3+O2 ��n
2 2 1
n m 0.1mol-0.09mol=0.01mol
n=m=0.02mol��
1����CO2��ȫ��Ӧ�����һ������CO2��Ϊ0.02mol��O2Ϊ0.1mol-0.02mol=0.08mol
2 Na2O2+4 HCl=4NaCl+2H2O+O2
0.16mol 0.32mol 0.08mol
NaHCO3+HCl=NaCl+H2O+CO2
0.02mol 0.02mol 0.02mol
����Na2O2Ϊ0.16mol��NaHCO3Ϊ0.02mol���������Na2O2��NaHCO3�����ʵ���֮��0.16mol��0.02mol=8��1��
���ĵ�HClΪ0.32mol+0.02mol=0.34mol�����ʵ���Ũ��Ϊ
 =3.4mol/L��
=3.4mol/L��2����CO2δ��ȫ��Ӧ����Na2O2Ӧ��0.02 mol��
2Na2O2+4HCl=4NaCl+2H2O+O2
0.02mol 0.04mol 0.01mol
���һ������O2��Ϊ0.01mol������CO2Ϊ0.1mol-0.01mol=0.09mol��
NaHCO3+HCl=NaCl+H2O+CO2
0.09mol 0.09mol 0.09mol
����Na2O2Ϊ0.02mol��NaHCO3Ϊ0.09mol���������Na2O2��NaHCO3�����ʵ���֮��0.02mol��0.09mol=2��9��
���ĵ�HClΪ 0.04mol+0.09mol=0.13mol�����ʵ���Ũ��Ϊ
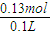 =1.3mol/L��
=1.3mol/L����ѡ��D��
���������⿼������ļ��㣬�ѶȽϴؼ��Ƕ�����̼��������Ƶķ�Ӧ���۽��м��㣮

��ϰ��ϵ�д�
�����Ŀ
��һ������Na2O2��NaHCO3�Ļ�Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ���100mLϡ������ǡ����ȫ��Ӧ�����ɵ������������Ϊ2.24L����״���£����ٽ�������ͨ����һ�ݻ�����У���ַ�Ӧ�õ�O2 2.016L����״���£�����ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ϡ��������ʵ���Ũ���ǣ�������
|
��2012?�Ϻ�ģ�⣩��һ������Na2O2��NaHCO3��Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ��뵽100mLϡ������ǡ����ȫ��Ӧ�����ɸ��������2.24L����״�������ٽ�������ͨ�뵽��һ�ݻ�����У���ַ�Ӧ���������Ϊ2.016L����״��������ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ԭϡ��������ʵ���Ũ�ȿ����ǣ�������
|
��һ������Na2O2��NaHCO3�Ļ�Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ���100mlϡ������ǡ����ȫ��Ӧ�����ɵ������������Ϊ2.24L����״���£����ٽ�������ͨ����һ�ݻ�����У���ַ�Ӧ�õ�O2 2.016L����״���£��������ϡ��������ʵ���Ũ���ǣ�������
| A��3.4mol?L-1 | B��0.2mol?L-1 | C��1.8mol?L-1 | D��3.6 mol?L-1 |
��һ������Na2O2��NaHCO3��Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ��뵽100mLϡ������ǡ����ȫ��Ӧ�����ɸ��������2��24L����״�������ٽ�������ͨ�뵽��һ�ݻ�����У���ַ�Ӧ���������Ϊ2��016L����״��������ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ԭϡ��������ʵ���Ũ�ȿ����ǡ��������������������� �� ��
|
| A | B | C | D |
| Na2O2��NaHCO3�����ʵ���֮�� | 8:1 | 9:2 | 1:8 | 2:9 |
| ԭϡ��������ʵ���Ũ�ȣ�mol/L�� | 3��4 | 1��1 | 1��8 | 1��3 |
��һ������Na2O2��NaHCO3��Ϸ�ĩ��Ϊ���ȷݣ�������һ�ݼ��뵽100mLϡ������ǡ����ȫ��Ӧ�����ɸ��������2��24L����״�������ٽ�������ͨ�뵽��һ�ݻ�����У���ַ�Ӧ���������Ϊ2��016L����״��������ԭ��Ϸ�ĩ��Na2O2��NaHCO3�����ʵ���֮�ȼ�ԭϡ��������ʵ���Ũ�ȿ����ǣ� ��
| | A | B | C | D |
| Na2O2��NaHCO3�����ʵ���֮�� | 8:1 | 9:2 | 1:8 | 2:9 |
| ԭϡ��������ʵ���Ũ�ȣ�mol/L�� | 3��4 | 1��1 | 1��8 | 1��3 |