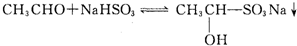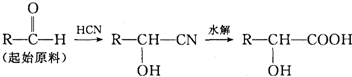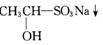��Ŀ����
��֪�ʻ��������뱥��NaHSO3��Һ���Է������·�Ӧ��
![]()
��1���ʻ�������ͱ���NaHSO3�ķ�Ӧ�������£�
| �ʻ������� | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ���ʣ�1Сʱ�ڣ� | 88.7 | 56.2 | 36.4 | 23.4 |
| �ʻ������� | ��CH3��2CHCOCH3 | ��CH3��3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ���ʣ�1Сʱ�ڣ� | 12.3 | 5.6 | 2 | 1 |
�ɼ���ȡ�������ʻ��������NaHSO3��Ӧ��Ӱ���У�д��3�����ɣ�
��
��
��
��2���������Ͽ��淴Ӧ���Է���ȩ��ͪ�Ļ�����д����ʹȩ��NaHSO3���ɵij��������ܽ���Լ��Ļ�ѧʽ ��д��2�֣����ڲ�ͬ�������ʡ���
��3�������ͪ��RCOCH3��ͨ���õ�����2����Ӧ������������ˮ���ȷ¡�
![]()
��д����һ����Ӧ�Ļ�ѧ����ʽ
��д��A�Ľṹ��ʽ
��4������ȩ��Ũ�������·�Ӧ���ɱ������κͱ��״����˷�Ӧ�������� ��
��1��ȩ��ͪ�������÷�Ӧ������ͪ���ѷ����÷�Ӧ���ʻ�������̼����Խ�٣�ȡ����Խ�࣬��ӦԽ�ѡ�
��2��HCl��NaOH����Na2CO3��
��3���� ��CH3��2CHCOCH3 + 3Cl2 + 3NaOH �� ��CH3��2CHCOCCl3 + 3NaCl + 3H2O
�� ��CH3��2CHCOONa
��4���绯��������ԭ��

��֪�ʻ��������뱥��NaHSO3��Һ���Է������·�Ӧ��
![]()
��1���ʻ�������ͱ���NaHSO3�ķ�Ӧ�������£�
| �ʻ������� | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ���ʣ�1Сʱ�ڣ� | 88.7 | 56.2 | 36.4 | 23.4 |
| �ʻ������� | ��CH3��2CHCOCH3 | ��CH3��3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ���ʣ�1Сʱ�ڣ� | 12.3 | 5.6 | 2 | 1 |
�ɼ���ȡ�������ʻ��������NaHSO3��Ӧ��Ӱ���У�д��3�����ɣ�
��
��
��
��2���������Ͽ��淴Ӧ���Է���ȩ��ͪ�Ļ�����д����ʹȩ��NaHSO3���ɵij��������ܽ���Լ��Ļ�ѧʽ ��д��2�֣����ڲ�ͬ�������ʡ���
��3�������ͪ��RCOCH3��ͨ���õ�����2����Ӧ������������ˮ���ȷ¡�
![]()
��д����һ����Ӧ�Ļ�ѧ����ʽ
��д��A�Ľṹ��ʽ
��4������ȩ��Ũ�������·�Ӧ���ɱ������κͱ��״����˷�Ӧ�������� ��