��Ŀ����
ij��ҵ�����л���һ�ֺ�ɫ���������������ʣ�Ϊ�˽����ʵijɷּ��ⶨ����Ĵ��ȣ���ѧ��ȤС���ͬѧ������ʵ���о������������̷����뽻�������������ϡ�
�����ij���������
���� | ��ѧʽ | ɫ��̬ | ��Ԫ�ص��������� |
�������� | FeO | ��ɫ��ĩ | w(Fe)=77.8% |
������ | Fe2O3 | ����ɫ��ĩ(�׳�����) | w(Fe)=70.0% |
���������� | Fe3O4 | ��ɫ����(�׳ƴ���������) | w(Fe)=72.4% |
�ڲ���(�Ҷ���H2C2O4)��Ũ������������ȷֽ�Ļ�ѧ����ʽΪH2C2O4![]() CO2��+ CO��+H2O
CO2��+ CO��+H2O
��ʵ������
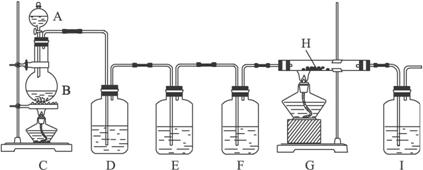
��ʵ���װ��ͼ����

��ҩƷ
a.���� b.��ҵ���� c.NaOH��Һ d.����ʯ��ˮ e.Ũ���� f.����
��ʵ�鼰���ݡ�
ȡ��ͬ��������Ʒ����ʵ�飬����ʵ���������£�
ʵ����� | ��Ʒ����/g | ������������/g |
1 | 4.00 | 2.91 |
2 | 8.00 | 7.00 |
3 | 10.00 | 7.27 |
4 | 12.00 | 8.72 |
5 | 14.00 | 10.18 |
6 | 16.00 | 11.63 |
�������������
(1)��ȤС���ͬѧ���Դ�����CO��ԭ�������������Ƶ�ʵ��װ����D��E��FӦ�ֱ�ʢ�ŵ��Լ�Ϊ____________��____________��___________(��д���)��������������____________��____________��_____________������װ�û��в�����֮�����㽨��Ľ��Ĵ�ʩ��________________________��
(2)��ʵ�����ݲ��ѵó�����һ��ʵ�����ݲ��ɿ�������������____________(�����)���ù�ҵ�����������ʵĻ�ѧʽΪ________________________��
(1)c d e(��д���) ����CO2 ��֤CO2�Ƿ���� ��ˮ(��������) ������װ�õ�β�����ڴ���һȼ�ŵľƾ���(���������ռ�β�����Ե��ܽ�β������C���ƾ��Ƶ�ȼ)
(2)2 FeO
������(1)������֪����ʵ������У�Cװ���ڲ���ֽ����CO��CO2��Dװ����ʢNaOH��Һ������CO2��Eװ����ʢ����ʯ��ˮ������CO2�Ƿ������Fװ����ʢŨ���ᣬ�������壻��Hװ����CO��ԭ���������
����CO�ж�����Ⱦ����������������װ�õ�β�����ڴ���һȼ�ŵľƾ��ƣ����������ռ�β������β������C���ƾ��Ƶ�ȼ��
(2)��������ʵ�����ݣ���2��ʵ�飬1 g��Ʒ������Լ0.88 g�����������ʵ���ԼΪ0.73 g���ʵ�2�����ݲ��ɿ�������Ʒ��w(Fe)=![]() ��100%=73%����ϳ�����������w(Fe)���ж�����ΪFeO��
��100%=73%����ϳ�����������w(Fe)���ж�����ΪFeO��

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д��۲������ϣ�
�����ij���������
����������FeO����ɫ���壬w(Fe)��77.8������������Fe2O3����ɫ���壬�׳����죬w(Fe)��70.0����������������Fe3O4����ɫ���壬�׳ƴ�����������w(Fe)��72.4����
�ڲ��ᣨ�Ҷ���H2CO4����ŨH2SO4���������ȷֽ�Ļ�ѧ����ʽΪ��
H![]() CO2��+CO��+H2O
CO2��+CO��+H2O
��ʵ������
��ʵ���װ��ͼ����
��ҩƷ���Լ�
a.���� b.��ҵ���� c.NaOH��Һ d.����ʯ��ˮ e.ŨH2SO
��ʵ�鼰���ݣ�ȡ��ͬ��������Ʒ����ʵ�飬����ʵ���������£�
ʵ����� | ��Ʒ����/g | ������������/g |
1 | 4.00 | 2.91 |
2 | 8.00 | 7.00 |
3 | 10.00 | 7.27 |
4 | 12.00 | 8.72 |
5 | 14.00 | 10.18 |
6 | 16.00 | 11.63 |
�������������
��1����ȤС���ͬѧ���Դ�����CO��ԭ�������������Ƶ�ʵ��װ����D��E��FӦ�ֱ�ʢ�ŵ��Լ�Ϊ______________��______________��______________����д��ţ���ͬ����������������____________________________������װ�û��в�����֮�����㽨��Ľ���ʩ��__________________________________________��
��2����ʵ�����ݲ��ѵó�����һ��ʵ�����ݲ��ɿ�������������______________������ţ����ù�ҵ�������������ʵĻ�ѧʽΪ______________��
��3���ù�ҵ������w��Fe2O3����______________��