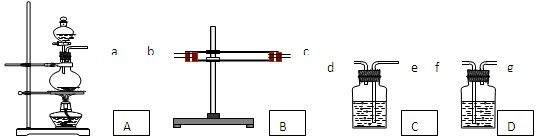
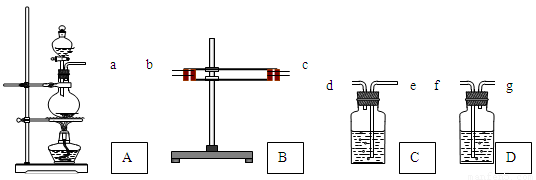
��Ŀ����
Ϊ����֤Cu�� ŨH2SO4��Ӧ�IJ�������SO2��H2O��ѡ����ͼ��ʾ����(�ں�����)��װ��ʵ��װ�� . B������ˮ����ͭ��C����Ʒ�졢D��������������Һ
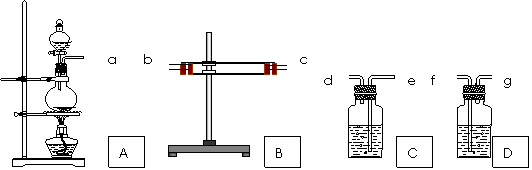
(1)�簴������������������������װ�õ���ȷ˳����(����ӿ���ĸ)��a�������� ������ �������� �������� �������� ��
(2) ����B��CӦ��������ʵ������ű����Ѽ����SO2��H2O?
B���������������� ��C���������������� ��
(3)D������������Һ������������������������������������������
(4)д��A�з�Ӧ�Ļ�ѧ����ʽ������������������������������������ .
��1��b�� c�� e���� d���� f���� (2��)
��2����ɫ��������ɫ(2��)�������� Ʒ����ɫ(2��)
��3�������(2��)
��4�� Cu+2H2SO4 CuSO4+SO2+H2O(2��)

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ