��Ŀ����
����Ŀ������������
(1) 2-��������ȥ��Ӧ(��ѧ����ʽ)______________________________________��
(2) 1,2-���������ˮ��(��ѧ����ʽ)_______________________________________����
(3)�����㶹��( )�������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹��(
)�������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹��( )��Ҫ�õ����Լ��У�NaOH��Һ��_________________��
)��Ҫ�õ����Լ��У�NaOH��Һ��_________________��
(4)ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź������ױ���������ֻ��һ�����͵���ԭ�ӡ���A�Ľṹ��ʽΪ______________________��A�Ƿ����˳���칹�壿________ (������������������)��
���𰸡� CH3-CHBr-CH3+NaOH![]() CH3-CH=CH2��+NaBr+H2O CH2BrCH2Br+2NaOH
CH3-CH=CH2��+NaBr+H2O CH2BrCH2Br+2NaOH![]() CH2OHCH2OH+2NaBr ϡ���ᣬ�Ȼ�����Ũ��ˮ
CH2OHCH2OH+2NaBr ϡ���ᣬ�Ȼ�����Ũ��ˮ ![]() ��
��
��������������Ҫ����±�������л�������ʡ�
(1) 2-��������ȥ��Ӧ�Ļ�ѧ����ʽ��CH3-CHBr-CH3+NaOH![]() CH3-CH=CH2��+NaBr+H2O��
CH3-CH=CH2��+NaBr+H2O��
(2) 1,2-���������ˮ�ⷴӦ�Ļ�ѧ����ʽ��CH2BrCH2Br+2NaOH![]() CH2OHCH2OH+2NaBr��
CH2OHCH2OH+2NaBr��
(3)�����㶹��(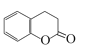 )ˮ��������ǻ�������һ��ͬ���칹��(
)ˮ��������ǻ�������һ��ͬ���칹��(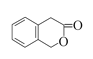 )ˮ��������ǻ�������ʹ����������������Һ������ˮ�⣬������ϡ����ָ�����ԭ���ţ����ʹ���Ȼ�����Һ��Ũ��ˮ���Լ�����Һ������ɫ�������ɫ�������Ƕ����㶹�ء������Լ��У�NaOH��Һ��ϡ���ᡢ�Ȼ�����Һ��Ũ��ˮ��
)ˮ��������ǻ�������ʹ����������������Һ������ˮ�⣬������ϡ����ָ�����ԭ���ţ����ʹ���Ȼ�����Һ��Ũ��ˮ���Լ�����Һ������ɫ�������ɫ�������Ƕ����㶹�ء������Լ��У�NaOH��Һ��ϡ���ᡢ�Ȼ�����Һ��Ũ��ˮ��
(4)ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫��������A�ķ���ʽΪC6H12���˴Ź������ױ���������ֻ��һ�����͵���ԭ�ӡ���A�Ľṹ��ʽΪ![]() ��A��˫��̼ԭ�������ɷ���ȫ��ͬ������A������˳���칹�塣
��A��˫��̼ԭ�������ɷ���ȫ��ͬ������A������˳���칹�塣

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�����Ŀ��ijʵ��С���о���Һ��AgNO3��Na2S�ķ�Ӧ��
ʵ�� | �Լ� | ���� | |
| �Թ� | �ι� | |
��pH = 4�� |
��pH = 9�� | ���ֺ�ɫ���� | |
��1�������ӷ���ʽ����Na2S��ҺpH > 7��ԭ��________��
��2��ʵ��С��ͬѧ��Ϊ��ɫ�����п��ܺ���Ag2O��Ag2S��Ag�����ʵ����֤��
��֪��i��Ũ�����ܽ�Ag2Sת��Ϊ![]() ��
��![]() ��
��
ii��Ag2O���ܽ���Ũ��ˮ���γ�������Һ����Ag2S��Ag�����ܡ�
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ�����к���Ag2S��

�Լ�1���Լ�2�ֱ���_________��_________��
����1������2�ֱ���_________��_________��
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ������������Ag2O����ʵ�����������������
ʵ����� | ʵ������ | |
����i | ȡ����������Һ�������еμ����� | ���ְ�ɫ���� |
����ii | ȡ����ϴ�Ӻ�ĺ�ɫ������____________ | ____________ |
�� �����飬����������Ag��

��3��ʵ��С��ͬѧ��ΪAgNO3��Һ���������ԣ���һ���������ܹ�����Na2S�����ʵ������о���ʵ��װ������ͼ��ʾ������õ�ѹΪa��![]() ����
����
��AgNO3��Һ������![]() �����ʽ����Ʋ⣺
�����ʽ����Ʋ⣺
����1�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
����2�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
������ͼװ�ü����о�����֪����ѹ��С��ӳ������������ԭ��ǿ���IJ��죻�����������뻹ԭ��ǿ������Խ��ѹԽ��
�� ��![]() ��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��
��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��![]() ����
����
�� ����ʵ��֤ʵ������![]() ��������һ������
��������һ������![]() ����֤����______��
����֤����______��
ʵ����ۣ�AgNO3��Һ��Na2S��Һ�ķ�Ӧ�����뷴Ӧ�����йء�




