��Ŀ����
��10��)����A��B��C��D���ֿ�������,���ǵ������ӷֱ������Ba2+��Ag+��Na+��Cu2+�е�ijһ�֣��������������NO3-�� SO42-�� Cl-��CO32-�����е�ijһ�֡�
(1)�����������ܽ�����֧�Թ��У�ֻ��C����Һ����ɫ��
(2)����1������֧�Թ��зֱ�������ᣬB����Һ���г������ɣ�D����Һ������ɫ��ζ����ų���
��������ʵ����ʵ�ƶ����ǵĻ�ѧʽΪ��A B C D
д��B�������ᷴӦ�����ӷ���ʽ
(1)�����������ܽ�����֧�Թ��У�ֻ��C����Һ����ɫ��
(2)����1������֧�Թ��зֱ�������ᣬB����Һ���г������ɣ�D����Һ������ɫ��ζ����ų���
��������ʵ����ʵ�ƶ����ǵĻ�ѧʽΪ��A B C D
д��B�������ᷴӦ�����ӷ���ʽ
C
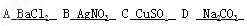


��ϰ��ϵ�д�
�����Ŀ
 ��Һ���ų����塣
��Һ���ų����塣