题目内容
【题目】LiCoO2用途广泛,如可作为锂离子电池的电极。回答下列问题。
(1)在周期表中,与Li的化学性质最相似的邻族元素是_________,该元素基态原子核外M层电子的自旋状态_________(填“相同”或“相反”)。
(2)[Co(NH3)6]Cl3是橙黄色晶体,该配合物中提供空轨道接受孤对电子的微粒是___,配体分子的价层电子对互斥模型为___,写出一种与配体分子互为等电子体的分子_________(填分子式)。
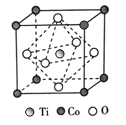
(3)某钴化合物纳米粉可以提高碱性电池的性能。该化合物晶胞结构如图所示,则该钴化合物的化学式为______,与Co原子等距离且最近的O原子个数为______。
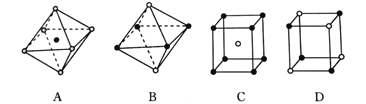
(4)Li还可形成多种物质。二异丙基胺基锂( )是有机合成中常用的物质,氮原子的杂化方式为_________。Li+可以镶嵌在C60中,形成的[LiC60]PF6与NaCl具有类似的晶胞结构,下面是从某晶体结构中分割出来的部分结构图,其中属于从[LiC60]PF6晶体中分割出来的结构图是___。
)是有机合成中常用的物质,氮原子的杂化方式为_________。Li+可以镶嵌在C60中,形成的[LiC60]PF6与NaCl具有类似的晶胞结构,下面是从某晶体结构中分割出来的部分结构图,其中属于从[LiC60]PF6晶体中分割出来的结构图是___。
(5)金属锂晶体的结构为体心立方密堆积,晶胞边长为351 pm,则锂晶体中原子的空间利用率为_________(列出含π的计算式)。
【答案】Mg 相反 Co3+ 四面体形 PH3(或AsH3) CoTiO3 12 sp3 AD ![]() ×100%
×100%
【解析】
(1)根据对角线法则分析;
(2)在配位化合物中,配位体提供孤对电子,中心离子提供空轨道;根据价层电子对互斥理论判断配位体的空间构型,根据等电子体概念书写其相应的等电子体化学式;
(3)用均摊方法计算一个晶胞中含有的各种元素的原子数目,得到其化学式,根据空间分布计算一个Co原子等距离且最近的O原子个数;
(4)结合原子形成的轨道数目分析计算;结合NaCl的晶体结构分析判断;
(5)Li为体心立方结构,先计算出1个晶胞中含有的Li的原子数目,再根据晶胞体积及Li原子的体积计算锂晶体中原子的空间利用率。
(1)在周期表中,与Li的化学性质最相似的邻族元素是Mg,Mg是12号元素,原子核外电子排布为2、8、2,所以在价态Mg原子核外M层上有2个电子在同一轨道上,自旋方向相反;
(2)在配位化合物[Co(NH3)6]Cl3中,中心离子Co3+提供空轨道,接受孤电子对,配位体NH3的N原子提供孤电子对,NH3的中心原子N原子形成3个N-H键,还有一对孤对电子,价层电子对数为3+1=4,NH3的价层电子对互斥模型为四面体形;与NH3互为等电子体的分子为PH3或AsH3;
(3)根据物质的晶胞结构可知,Ti在晶体内,在一个晶胞中含有Ti原子1个;Co原子位于晶胞的顶点上,1个晶胞中含有的Co原子数目为8×![]() =1,O原子在晶胞各个面的面心上,含有的O原子数目为6×
=1,O原子在晶胞各个面的面心上,含有的O原子数目为6×![]() =3,所以该晶体化学式为CoTiO3;在CoTiO3晶体中,1个Co原子周围等距离且最近的O原子个数为(3×8)÷2=12个;
=3,所以该晶体化学式为CoTiO3;在CoTiO3晶体中,1个Co原子周围等距离且最近的O原子个数为(3×8)÷2=12个;
(4)N原子形成了3个共价键,还有1对孤对电子,价层电子对数为3+1=4,所以杂化轨道类型为sp3杂化;在NaCl晶体中1个Na+周围有6个Cl-,1个Cl-周围有6个Na+。[LiC60]PF6与NaCl具有类似的晶胞结构,所以1个[LiC60]+周围有6个PF6-,1个PF6-周围有6个[LiC60]+,故合理选项是AD;
(5)金属锂晶体的结构为体心立方密堆积,在一个晶胞中含有的Li原子个数为8×![]() +1=2,Li晶胞边长为351 pm,则Li原子半径r=
+1=2,Li晶胞边长为351 pm,则Li原子半径r=![]() ×351 pm,因此2个Li原子所占体积V(Li)=2×
×351 pm,因此2个Li原子所占体积V(Li)=2×![]() πr3=2×
πr3=2×![]() π
π![]() ,晶胞的体积V(晶胞)=
,晶胞的体积V(晶胞)=![]() ,所以Li晶胞中原子的空间占有率为
,所以Li晶胞中原子的空间占有率为![]() ×100%。
×100%。

 阅读快车系列答案
阅读快车系列答案