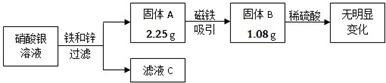
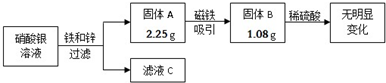
��Ŀ����
��15�֣�ijУ��ȤС��ͬѧ���о�SO2�����ʡ�
��1������صĺ������ʷ�Ϊ���±���ʾ3�飬��2��������X�Ļ�ѧʽ�� ��
| ��1�� | ��2�� | ��3�� |
| S�����ʣ� | SO2��X��Na2SO3��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |
��2��������ͼ��ʾ��װ���о�SO2�����ʣ�
���۵㣺SO2 ��76��1�棬SO3 16��8�棻�е㣺SO3 ��10�棬SO3 44��8�棩
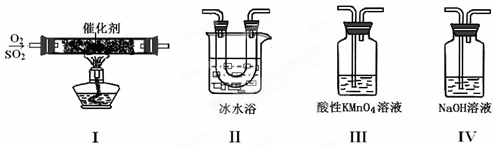
��װ��Iģ�ҵ������SO2�������ķ�Ӧ���仯ѧ����ʽ�� ��
�ڼ�ͬѧ��I��II��III��IV��˳������װ�ã�װ��II�������� ��װ��III����Һ����ɫ������Mn2+��ͬʱpH���ͣ���÷�Ӧ�����ӷ���ʽ�� ��
����ͬѧ��I��II��IV��˳������װ�ã���װ��IV����40 mL 2��5 mol?L��1 NaOH��Һ����Ӧ������4��8 g����װ��IV�з�����Ӧ���ܻ�ѧ����ʽ�� ��
��1����3�֣�H2SO3
��2���٣�3�֣�2SO2 + O2 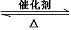 2SO3
2SO3
�ڣ�3�֣�ʹSO3����ɹ�����SO2����
��3�֣�5SO2 + 2H2O + 2MnO4��="=" 5SO42�� + 2Mn2+ + 4H+
�ۣ�3�֣�3SO2 + 4NaOH ="=" Na2SO3 + 2NaHSO3 + H2O
����

 ��У����ϵ�д�
��У����ϵ�д���15�֣�ijУ��ȤС��ͬѧ���о�SO2�����ʡ�
��1������صĺ������ʷ�Ϊ���±���ʾ3�飬��2��������X�Ļ�ѧʽ�� ��
| ��1�� | ��2�� | ��3�� |
| S�����ʣ� | SO2��X��Na2SO3��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |
��2��������ͼ��ʾ��װ���о�SO2�����ʣ�
���۵㣺SO2 ��76��1�棬SO3 16��8�棻�е㣺SO2 ��10�棬SO3 44��8�棩
��װ��Iģ�ҵ������SO2�������ķ�Ӧ���仯ѧ����ʽ�� ��
�ڼ�ͬѧ��I��II��III��IV��˳������װ�ã�װ��II�������� ��װ��III����Һ����ɫ������Mn2+��ͬʱpH���ͣ���÷�Ӧ�����ӷ���ʽ�� ��
����ͬѧ��I��II��IV��˳������װ�ã���װ��IV����40 mL 2��5 mol•L��1 NaOH��Һ����Ӧ������4��8 g����װ��IV�з�����Ӧ���ܻ�ѧ����ʽ�� ��
��15�֣�ijУ��ȤС��ͬѧ���о�SO2�����ʡ�
��1������صĺ������ʷ�Ϊ���±���ʾ3�飬��2��������X�Ļ�ѧʽ�� ��
|
��1�� |
��2�� |
��3�� |
|
S�����ʣ� |
SO2��X��Na2SO3��NaHSO3 |
SO3��H2SO4��Na2SO4��NaHSO4 |
��2��������ͼ��ʾ��װ���о�SO2�����ʣ�
���۵㣺SO2 ��76��1�棬SO3 16��8�棻�е㣺SO2 ��10�棬SO3 44��8�棩

��װ��Iģ�ҵ������SO2�������ķ�Ӧ���仯ѧ����ʽ�� ��
�ڼ�ͬѧ��I��II��III��IV��˳������װ�ã�װ��II�������� ��װ��III����Һ����ɫ������Mn2+��ͬʱpH���ͣ���÷�Ӧ�����ӷ���ʽ�� ��
����ͬѧ��I��II��IV��˳������װ�ã���װ��IV����40 mL 2��5 mol•L��1 NaOH��Һ����Ӧ������4��8 g����װ��IV�з�����Ӧ���ܻ�ѧ����ʽ�� ��