��Ŀ����
�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�
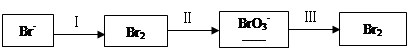
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
��ԭ��Һ��NaI�����ʵ���Ϊ______mol��
��1����ˮ�����������Ԫ�صı仯���£�

�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2��
 Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
��1���٢�.Cl2��2Br-��Br2��2Cl-
��.Cl2��H2O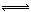 H+��Cl-��HClO�� ����c(H+)��ƽ�������ƶ�������Cl2��ˮ��Ӧ
H+��Cl-��HClO�� ����c(H+)��ƽ�������ƶ�������Cl2��ˮ��Ӧ
��3 3 1 3 5 NaBr
�������л�ԭ�ԣ����������Ӧ
��2��3
��.Cl2��H2O
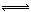 H+��Cl-��HClO�� ����c(H+)��ƽ�������ƶ�������Cl2��ˮ��Ӧ
H+��Cl-��HClO�� ����c(H+)��ƽ�������ƶ�������Cl2��ˮ��Ӧ��3 3 1 3 5 NaBr
�������л�ԭ�ԣ����������Ӧ
��2��3
�����������1���٢�.ͨ��������Cl2��Br?��Ӧ��Cl2��2Br-��Br2��2Cl-
��.Cl2��ˮ�ķ�ӦΪ���淴Ӧ��Cl2��H2O
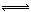 H+��Cl-��HClO��������Һ��pH������c(H+)��ƽ�������ƶ�������Cl2��ˮ��Ӧ��
H+��Cl-��HClO��������Һ��pH������c(H+)��ƽ�������ƶ�������Cl2��ˮ��Ӧ���ڸ��ݻ��ϼ۵ı仯��Ԫ���غ����֪������ΪNaBr���л��ϼ�����������ƽ��ѧ����ʽ��
��HCl��ClԪ��Ϊ-1�ۣ�Ϊ��ͼ�̬������������л�ԭ�ԣ��ܱ������������������������١�
��2������������ԭ��Ӧ�е�ʧ��������ȣ��ɵ�5��2.6mol=2��0.5mol+6��n(IO3?)����n(IO3?)=2mol������Ԫ���غ㣬ԭ��Һ��n(NaI)=0.5mol��2+2mol=3mol��

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
�����Ŀ
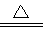 Cu2��+ SO2��+ 2H2O
Cu2��+ SO2��+ 2H2O H2��+C12��
H2��+C12��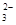 + 2H+== CO2 �� + H2O
+ 2H+== CO2 �� + H2O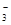 == Cu2+ + NO�� + H2O
== Cu2+ + NO�� + H2O