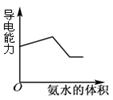
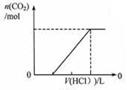
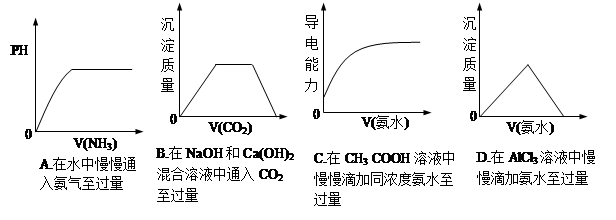��Ŀ����
�������ֿ���������A��B��C��D��E���������������������ӻ�����ͬ���ֱ�������������Na+��Al3+��Mg2+��Ba2+��Fe3+������������Cl?��OH?��NO3?��CO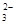 ��X�е�һ�֡�
��X�е�һ�֡�
��1��ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������
���ѧʽ����
��2��Ϊ��ȷ��X���ֽ���1���е��������ʼ�ΪA��B����C��B����Һ���ʱ���������ɫ��������ɫ��ζ���壻��C��A����Һ���ʱ�����ػ�ɫ��������ó����е���ϡHNO3�����������ܽ⣬������а�ɫ���������ܽ⡣��
��XΪ ��
A��SO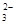 B��SO
B��SO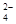 C��CH3COO D��SiO
C��CH3COO D��SiO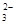
��A�еĻ�ѧ������Ϊ ��
�۽�0.02 mol��A��0.01mol��Cͬʱ�ܽ�������������ˮ�У���ַ�Ӧ���������ó���������Ϊ ����ȷ��0.1g)��
�����������Ѿ�ȷ�������ʣ����Լ����D��E�е������ӡ������ʵ��������衢������ ��
��3����CuͶ�뵽װ��D��Һ���Թ��У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣���÷�Ӧ�����ӷ���ʽΪ�� ��
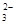 ��X�е�һ�֡�
��X�е�һ�֡���1��ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������
���ѧʽ����
��2��Ϊ��ȷ��X���ֽ���1���е��������ʼ�ΪA��B����C��B����Һ���ʱ���������ɫ��������ɫ��ζ���壻��C��A����Һ���ʱ�����ػ�ɫ��������ó����е���ϡHNO3�����������ܽ⣬������а�ɫ���������ܽ⡣��
��XΪ ��
A��SO
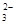 B��SO
B��SO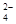 C��CH3COO D��SiO
C��CH3COO D��SiO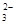
��A�еĻ�ѧ������Ϊ ��
�۽�0.02 mol��A��0.01mol��Cͬʱ�ܽ�������������ˮ�У���ַ�Ӧ���������ó���������Ϊ ����ȷ��0.1g)��
�����������Ѿ�ȷ�������ʣ����Լ����D��E�е������ӡ������ʵ��������衢������ ��
��3����CuͶ�뵽װ��D��Һ���Թ��У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣���÷�Ӧ�����ӷ���ʽΪ�� ��
��1��Na2CO3��Ba(OH)2��2�֣�
��2����B ��2�֣�
�����Ӽ��������ԣ����ۼ���2�֣�
��6.1g ��2�֣�
����D����Һ������Ba(OH)��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬
��D���Al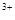 ��E���Mg
��E���Mg ��
��
����D����Һ�м�������NaCO��Һ���������˰�ɫ��������ɫ��ζ�����壬��D�к���Al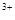 ��E�к���Mg
��E�к���Mg ����2�֣�����������Ҳ�÷֣�
����2�֣�����������Ҳ�÷֣�
��3��3Cu+8H++2NO3?=3Cu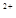 +2NO��+4H2O ��2�֣�
+2NO��+4H2O ��2�֣�
��2����B ��2�֣�
�����Ӽ��������ԣ����ۼ���2�֣�
��6.1g ��2�֣�
����D����Һ������Ba(OH)��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬
��D���Al
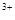 ��E���Mg
��E���Mg ��
������D����Һ�м�������NaCO��Һ���������˰�ɫ��������ɫ��ζ�����壬��D�к���Al
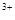 ��E���Mg
��E�к���Mg ����2�֣�����������Ҳ�÷֣�
����2�֣�����������Ҳ�÷֣���3��3Cu+8H++2NO3?=3Cu
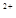 +2NO��+4H2O ��2�֣�
+2NO��+4H2O ��2�֣������������1��CO32-��Al3+��Mg2+��Ba2+��Fe3+���ܴ������棬OH-��Al3+��Mg2+��Fe3+���ܴ������棬�����������������ӻ�����ͬ�����Զ�Ӧ������ӦΪNa2CO3��Ba��OH��2������������������飬�ض����С�
��2���ٽ� ��1���е��������ʼ�ΪA��B����C��B����Һ���ʱ���������ɫ��������ɫ��ζ���壬���ɫ����ΪFe��OH��3������ΪCO2��Ӧ�������ǻ���ˮ�ⷴӦ��2Fe3++3CO32-+3H2O=2Fe��OH��3��+3CO2������BΪNa2CO3��AΪBa��OH��2��C�к���Fe3+����C��A����Һ���ʱ������������ó����е������ϡHNO3�����������ܽ⣬������а�ɫ���������ܽ⣬˵������BaSO4��������CΪFe2��SO4��3��SO32-��SiO32-��Fe3+���ܹ��棬CH3COO-���������ɱ��γ�������Xֻ��ΪSO42-����B����ȷ��
��AΪBa(OH)2���������Ӽ��ͼ��Թ��ۼ���
��Ba(OH)2���ʵ���Ϊ0.02mol��Fe2(SO4)3�����ʵ���Ϊ0.01mol�����ݷ���ʽ��֪Fe2(SO4)3���������ɵ�Fe(OH)3Ϊ0.02mol��2��1/3��107g/mol,���ɵ�BaSO4Ϊ0.02mol��233g/mol����6.1g��
��D�к���NO3-����E�к���Cl-��ʣ��Al3+��Mg2+��Al��OH��3�����ڹ���Ba��OH��2��Һ�����鷽��Ϊ��ȡ����D����Һ���Թ��У�����Ba��OH��2��Һ���������ȳ��ְ�ɫ�������ܽ⣬��D�к���Al3+�������ɵİ�ɫ�������ܽ⣬��D�к���Mg2+��
��3����CuͶ��D��Һ�У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣�������ΪNO2��˵��D�к���NO3-����Ӧ���ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��

��ϰ��ϵ�д�
�����Ŀ