��Ŀ����
��8�֣�����S2-��SO32-��NH4+��Al3+��HPO42-��Na+��SO42-��AlO2-��Fe3+��HCO3-��Cl-�����ӣ��밴Ҫ����գ�
��1����ˮ��Һ�У�������ˮ��ʼ��Ե��� ��
��2����ˮ��Һ�У�������ˮ������Ե��� ��
��3�����������Խ�ǿ����Һ��������ڣ������ڼ��Խ�ǿ����Һ��������ڵ������� ��
��4���Ȳ��������Խ�ǿ����Һ��������ڣ��ֲ����ڼ��Խ�ǿ����Һ��������ڵ������� ��
��1����ˮ��Һ�У�������ˮ��ʼ��Ե��� ��
��2����ˮ��Һ�У�������ˮ������Ե��� ��
��3�����������Խ�ǿ����Һ��������ڣ������ڼ��Խ�ǿ����Һ��������ڵ������� ��
��4���Ȳ��������Խ�ǿ����Һ��������ڣ��ֲ����ڼ��Խ�ǿ����Һ��������ڵ������� ��
��1��S2-��SO32-��HPO42-��AlO2-��HCO3- ��2��NH4+��Al3+��Fe3+
��3��Na+��SO42-��Cl- ��4��HPO42-��HCO3-
��3��Na+��SO42-��Cl- ��4��HPO42-��HCO3-
��1����������ˮ���Լ��ԣ�������S2-��SO32-��HPO42-��AlO2-��HCO3-��
��2���������������ˮ�������ԣ�������NH4+��Al3+��Fe3+��
��3���������Ӽ�OH��������Ӧ����ǿ��������ǿ��������ӣ���Na+��SO42-��Cl-��
��4���������ʽ�μȺ������ӣ�Ҳ��OH����Ӧ��������HPO42-��HCO3-��
��2���������������ˮ�������ԣ�������NH4+��Al3+��Fe3+��
��3���������Ӽ�OH��������Ӧ����ǿ��������ǿ��������ӣ���Na+��SO42-��Cl-��
��4���������ʽ�μȺ������ӣ�Ҳ��OH����Ӧ��������HPO42-��HCO3-��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
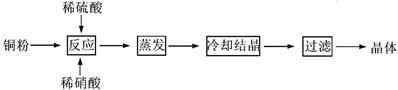
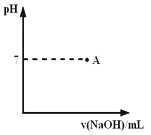

 ����֪ij������Һ�����µ�AG=12�����ڴ���Һ���ܴ��������������
����֪ij������Һ�����µ�AG=12�����ڴ���Һ���ܴ�������������� ��
��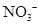 ��
��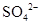 ��
��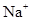
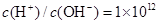 ����Һ��
����Һ��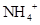 ��
��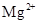 ��
��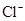 ��
��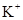
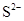 ��
��