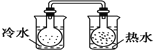
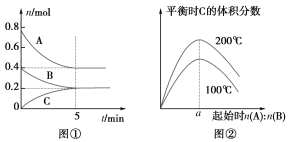
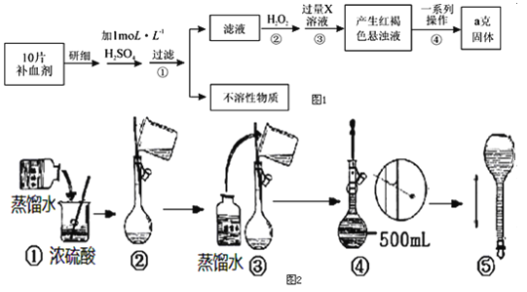
��Ŀ����
����Ŀ����1 200 ��ʱ����Ȼ���������лᷢ�����з�Ӧ
H2S(g)��3/2O2(g)===SO2(g)��H2O(g)����H1 ��
2H2S(g)��SO2(g)==3/2S2(g)��2H2O(g)����H2 ��
H2S(g)��1/2O2(g)===S(g)��H2O(g)����H3 ��
2S(g)===S2(g)����H4
��H4����ȷ����ʽΪ(����)
A. ��H4��2/3(��H1����H2��3��H3) B. ��H4��2/3(3��H3����H1����H2)
C. ��H4��2/3(��H1����H2��2��H3) D. ��H4��2/3(��H1����H2��3��H3)
���𰸡�A
��������
���ø�˹���ɷ��������ܻ�ѧ��Ӧ��һ����ּ�����ɣ��䷴Ӧ���Dz���ģ�����Ŀ�귽�̸�д�ַ��̣�Ȼ�������Ӧ�ȣ��ó����ۡ�
����Ŀ�귽��2S(g)===S2(g)����H4���ѷ��̢۷�д����ѧ����������2���ѷ��̢ڳ���![]() ���ѷ��̢ٳ���
���ѷ��̢ٳ���![]() ��Ȼ��������ӣ����õ�2S(g)===S2(g)�����䷴Ӧ����H4 = ��H3��2+��H2��
��Ȼ��������ӣ����õ�2S(g)===S2(g)�����䷴Ӧ����H4 = ��H3��2+��H2��![]() +��H1��
+��H1��![]() = 2/3(��H1����H2��3��H3)����A����ȷ��
= 2/3(��H1����H2��3��H3)����A����ȷ��
��ѡA��

��ϰ��ϵ�д�
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
�����Ŀ