��Ŀ����
ʵ��������NaNO3��������80 mL 1.4 mol��L-1��NaNO3��Һ���Իش�
(1)����������__________��
A.�ձ� B.500 mL����ƿ C.��Ͳ
D.��ͷ�ι� E.������ F.100 mL����ƿ
(2)����ʱӦ��ȡNaNO3__________g��
(3)ʵ�鿪ʼʱ����������ƿ__________��
(4)������һ�����ʵ���Ũ����Һ��ʵ���У����в�����������ҺŨ����Ӱ�����(д��ĸ) __________����ʹ������ҺŨ��ƫ�����__________����ʹ������ҺŨ��ƫС����__________��
A.���ձ����ܽ����ʣ�����ʱ��������������Һ
B.δ��ϴ���ձ��ڱڵ���Һת��������ƿ
C.����ƿ���������ҺҺ��δ���̶��߱�ֹͣ��ˮ
D.����õ���Һ������ƿת�Ƶ�����ྻ���Լ�ƿ��ʱ������������
E.���ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ
F.����ƿ��Һ�潫�ﵽ�̶���ʱ�����ӿ̶��ߺ�Һ��
(1) B (2) 11.9 (3) �Ƿ�©ˮ (4) D��E C��F A��B
���������������1������û��80ml������ƿ������Ӧ������100ml������Ҫ��������500ml����ƿ����ѡB��
��2������ʱӦ��ȡ�����ƹ����������0.1L��1.4mol/L��85g/mol��11.9g��
(3)ʵ�鿪ʼʱ����������ƿ�Ƿ�©ˮ��
��4������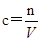 ��֪�����ձ����ܽ����ʣ�����ʱ��������������Һ�������ʵ��������٣�Ũ��ƫ�ͣ�δ��ϴ���ձ��ڱڵ���Һת��������ƿ��ͬ���������������٣�Ũ��ƫ�ͣ�����ƿ���������ҺҺ��δ���̶��߱�ֹͣ��ˮ��������ƿ����Һ������٣�Ũ��ƫ�ߣ�����õ���Һ������ƿת�Ƶ�����ྻ���Լ�ƿ��ʱ��������������Ũ�Ȳ��䣻���ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ������Ӱ��ʵ����������ƿ��Һ�潫�ﵽ�̶���ʱ�����ӿ̶��ߺ�Һ�棬������ƿ����Һ������٣�Ũ��ƫ�ߡ�
��֪�����ձ����ܽ����ʣ�����ʱ��������������Һ�������ʵ��������٣�Ũ��ƫ�ͣ�δ��ϴ���ձ��ڱڵ���Һת��������ƿ��ͬ���������������٣�Ũ��ƫ�ͣ�����ƿ���������ҺҺ��δ���̶��߱�ֹͣ��ˮ��������ƿ����Һ������٣�Ũ��ƫ�ߣ�����õ���Һ������ƿת�Ƶ�����ྻ���Լ�ƿ��ʱ��������������Ũ�Ȳ��䣻���ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ������Ӱ��ʵ����������ƿ��Һ�潫�ﵽ�̶���ʱ�����ӿ̶��ߺ�Һ�棬������ƿ����Һ������٣�Ũ��ƫ�ߡ�
���㣺����������ѡ��ʵ����������ʵ���Ũ�ȵ����ơ������Լ�������
�����������Ǹ߿��еij������ͣ������е��Ѷ�����Ŀ��飬���������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ������Ҫ�ǿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����ʵ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������������ѵ���������������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�������������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ�ݴ��жϡ�
