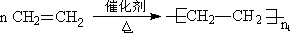��Ŀ����
��1��ij�л���Ľṹ��ʽΪHOOC-CH=CHOH��
��д�����л���ĺ��������ŵ����ƣ�
����֤���л����к���-COOH�����ų����õķ�����
��2��������ѡȡ�ʵ��Լ��Ʊ��������ᣬҪ������ѧ��Ӧ���Ⱥ�˳��д���йصķ�Ӧ����ʽ��ע����Ӧ������ָ����Ӧ����
ϡ���� ����������Һ ������Һ ���Ƶ�������ͭ����Һ
��д�����л���ĺ��������ŵ����ƣ�
�ǻ����Ȼ�
�ǻ����Ȼ�
������֤���л����к���-COOH�����ų����õķ�����
���л�����Һ�м���Na2CO3��ʯ����Һ�������ݲ�������Һ��죬��֤�����л��ﺬ-COOH
���л�����Һ�м���Na2CO3��ʯ����Һ�������ݲ�������Һ��죬��֤�����л��ﺬ-COOH
����2��������ѡȡ�ʵ��Լ��Ʊ��������ᣬҪ������ѧ��Ӧ���Ⱥ�˳��д���йصķ�Ӧ����ʽ��ע����Ӧ������ָ����Ӧ����
ϡ���� ����������Һ ������Һ ���Ƶ�������ͭ����Һ
��C6H10O5��n+nH2O
nC6H12O6
| ���� |
| �� |
��C6H10O5��n+nH2O
nC6H12O6
��Ӧ����| ���� |
| �� |
ˮ���ȡ����Ӧ
ˮ���ȡ����Ӧ
H2SO4+2NaOH=Na2SO4+2H2O
H2SO4+2NaOH=Na2SO4+2H2O
��Ӧ�����кͷ�Ӧ�ֽⷴӦ
�кͷ�Ӧ�ֽⷴӦ
CH2OH��CHOH��4CHO+2Cu��OH��2��Cu2O��+2H2O+CH2OH��CHOH��4COOH
CH2OH��CHOH��4CHO+2Cu��OH��2��Cu2O��+2H2O+CH2OH��CHOH��4COOH
��Ӧ����������Ӧ
������Ӧ
����������1���ٺ����������к���Oԭ�ӣ�
�������Ȼ������������飻
��2���Ʊ��������ᣬӦ�����õ���ˮ�����������ǣ����к������ԣ�Ȼ���������Ƶ�������ͭ����Һ�����������������������ᣮ
�������Ȼ������������飻
��2���Ʊ��������ᣬӦ�����õ���ˮ�����������ǣ����к������ԣ�Ȼ���������Ƶ�������ͭ����Һ�����������������������ᣮ
����⣺��1���л���Ľṹ��ʽΪHOOC-CH=CHOH������-COOH��-C=C-��-OH��
������������Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
�ڸ��л����к����Ȼ����������ԣ������л�����Һ�м���Na2CO3��ʯ����Һ�������ݲ�������Һ��죬��֤�����л��ﺬ-COOH��
�ʴ�Ϊ�����л�����Һ�м���Na2CO3��ʯ����Һ�������ݲ�������Һ��죬��֤�����л��ﺬ-COOH��
��2���Ʊ��������ᣬӦ�����õ���ˮ�����������ǣ����к������ԣ�Ȼ���������Ƶ�������ͭ����Һ�����������������������ᣬ
�漰�ķ�ӦΪ��C6H10O5��n+n H2O
n C6H12O6���÷�ӦΪˮ�ⷴӦ��ȡ����Ӧ��
���ۡ��������������� ������
H2SO4+2NaOH=Na2SO4+2H2O���÷�ӦΪ�кͷ�Ӧ�ֽⷴӦ��
CH2OH��CHOH ��4CHO+2Cu��OH��2��Cu2O��+2H2O+CH2OH��CHOH��4COOH���÷�ӦΪ������Ӧ��
�ʴ�Ϊ����C6H10O5��n+n H2O
n C6H12O6��ˮ�ⷴӦ��ȡ����Ӧ��H2SO4+2NaOH=Na2SO4+2H2O���кͷ�Ӧ�ֽⷴӦ��CH2OH��CHOH ��4CHO+2Cu��OH��2��Cu2O��+2H2O+CH2OH��CHOH��4COOH��������Ӧ��
������������Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
�ڸ��л����к����Ȼ����������ԣ������л�����Һ�м���Na2CO3��ʯ����Һ�������ݲ�������Һ��죬��֤�����л��ﺬ-COOH��
�ʴ�Ϊ�����л�����Һ�м���Na2CO3��ʯ����Һ�������ݲ�������Һ��죬��֤�����л��ﺬ-COOH��
��2���Ʊ��������ᣬӦ�����õ���ˮ�����������ǣ����к������ԣ�Ȼ���������Ƶ�������ͭ����Һ�����������������������ᣬ
�漰�ķ�ӦΪ��C6H10O5��n+n H2O
| ���� |
| �� |
���ۡ��������������� ������
H2SO4+2NaOH=Na2SO4+2H2O���÷�ӦΪ�кͷ�Ӧ�ֽⷴӦ��
CH2OH��CHOH ��4CHO+2Cu��OH��2��Cu2O��+2H2O+CH2OH��CHOH��4COOH���÷�ӦΪ������Ӧ��
�ʴ�Ϊ����C6H10O5��n+n H2O
| ���� |
| �� |
���������⿼���л���Ľṹ�����ʣ�����������Ʊ������ü��к���������Ϊ�����״��㣬��Ϥ�ṹ�����ʵĹ�ϵ���ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ