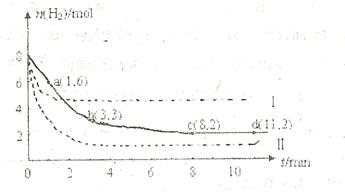
��Ŀ����
(8��)ij��Һ�п��ܺ���Ba2+��K+��Na+��SO42-��CO32-��HCO3-��Cl-��Br-�������е����ֻ��֡��ֽ�������ʵ�飺
��ȡ������Һ�����������BaCl2��Һ��������ɫ���������ˣ�������м�����������ᣬ������ȫ�ܽ⣬���������壻
����ʵ��ٵ���Һ�е���AgNO3��Һ�����ֵ���ɫ������
��ȡԭ��Һ������ɫ��Ӧ��ֱ�ӹ۲죬����ʻ�ɫ��
������ʵ���жϣ�����Һ�п϶����ڵ�������_________________________���϶������ڵ�����
Ϊ___________________������ȷ���Ƿ���ڵ�����Ϊ________________________��
���𰸡�
��8�֣����һ����1�֣��϶����ڣ�CO32����Br����Na+
�϶������ڣ�Ba2+��SO42��
����ȷ����K+��HCO3����Cl��
����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Ag��(aq)��Cl��(aq)
Ag��(aq)��Cl��(aq)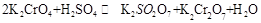
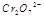 ��ˮ��Һ��Ϊ��ɫ��
��ˮ��Һ��Ϊ��ɫ��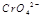 ��ˮ��Һ��Ϊ��ɫ��ij�����¸÷�Ӧ����ƽ�����ϵΪ�������ӵĻ��Һ����ɫΪ��ɫ��
��ˮ��Һ��Ϊ��ɫ��ij�����¸÷�Ӧ����ƽ�����ϵΪ�������ӵĻ��Һ����ɫΪ��ɫ��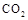 ,����Ч��������Դ�������ٿ����е��������塣��ҵ�������о�����
,����Ч��������Դ�������ٿ����е��������塣��ҵ�������о�����
 ����һ�ݻ�Ϊ2L���ܱ������У����H
����һ�ݻ�Ϊ2L���ܱ������У����H