��Ŀ����
��֪������A����Է�������Ϊ182.5���������ɿ��Ա�ʾΪCxHyOCl���й�ת����ϵ������ʾ��

C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ ����E��R�������ȡ�����ʶ�λ����֪R��R��Ϊ������
����E��R�������ȡ�����ʶ�λ����֪R��R��Ϊ������
��ش��������⣺
��1��A�к��еĺ��������ŵ�������
��2��д����C����E�Ļ�ѧ��Ӧ����
��3����֪��������R���л���R-OH����50%��һ��ȼ�ϵ���Ը�����Ϊȼ���ҵ������ҺΪ������Һ����õ�صĸ�����ӦΪ��
��4��D��Ũ���ᡢ���ȵ������·�Ӧ����F��C11H12O2����F��һ�������¿��Է����Ӿ۷�Ӧ��д���üӾ۷�Ӧ�Ļ�ѧ����ʽ��
 ��
��
��5����B�Ķ���ͬ���칹���У�д�����ӽṹ�к��� ������������ͬ���칹����
������������ͬ���칹���� ����дһ�֣�
����дһ�֣� ����дһ�֣���
����дһ�֣���

C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ
 ����E��R�������ȡ�����ʶ�λ����֪R��R��Ϊ������
����E��R�������ȡ�����ʶ�λ����֪R��R��Ϊ��������ش��������⣺
��1��A�к��еĺ��������ŵ�������
ȩ��
ȩ��
��A�Ļ�ѧʽΪC10H11OCl
C10H11OCl
����2��д����C����E�Ļ�ѧ��Ӧ����
���۷�Ӧ
���۷�Ӧ
����3����֪��������R���л���R-OH����50%��һ��ȼ�ϵ���Ը�����Ϊȼ���ҵ������ҺΪ������Һ����õ�صĸ�����ӦΪ��
2CH3OH+2H2O-12e-�T12H++2CO2
2CH3OH+2H2O-12e-�T12H++2CO2
����4��D��Ũ���ᡢ���ȵ������·�Ӧ����F��C11H12O2����F��һ�������¿��Է����Ӿ۷�Ӧ��д���üӾ۷�Ӧ�Ļ�ѧ����ʽ��


��5����B�Ķ���ͬ���칹���У�д�����ӽṹ�к���
 ������������ͬ���칹����
������������ͬ���칹����3
3
�֣�д����������һ�ֵĽṹ��ʽ�� ����дһ�֣�
����дһ�֣� ����дһ�֣�
����дһ�֣�������������A����Է�������Ϊ182.5���������ɿ��Ա�ʾΪCxHyOCl����12x+y=182.5-16-35.5=131������ʽӦΪC10H11OCl�����B�ܷ���������Ӧ��֪��A�к���-CHO��������-Cl��A�ڼ��������·���ˮ������C�����У�3����֪��������R���л���R-OH����50%��˵��R-OHӦΪCH3OH��C��CH3OH����������Ӧ����D��C11H14O3������C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ ����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ
����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮
������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮
 ����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ
����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮
������л���Ľṹ�������Լ������Ӧ��Ϣ�����⣮����⣺������A����Է�������Ϊ182.5���������ɿ��Ա�ʾΪCxHyOCl����12x+y=182.5-16-35.5=131������ʽӦΪC10H11OCl�����B�ܷ���������Ӧ��֪��A�к���-CHO��������-Cl��A�ڼ��������·���ˮ������C�����У�3����֪��������R���л���R-OH����50%��˵��R-OHӦΪCH3OH��C��CH3OH����������Ӧ����D��C11H14O3������C��һ�������¿ɷ�����Ӧ������һ�ָ߷��ӻ�����E��E�Ľṹ��ʽΪ ����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ
����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ����
����
��1�������Ϸ�����֪��A���еĺ���������Ϊȩ��������ʽΪC10H11OCl���ʴ�Ϊ��ȩ����C10H11OCl��
��2��CΪ ��E�Ľṹ��ʽΪ
��E�Ľṹ��ʽΪ ��C����E�������۷�Ӧ��
��C����E�������۷�Ӧ��
�ʴ�Ϊ�����۷�Ӧ��
��3����֪��������R���л���R-OH����50%��˵��R-OHӦΪCH3OH���Ը�����Ϊȼ���ҵ������ҺΪ������Һ����õ�صĸ�����ӦΪ2CH3OH+2H2O-12e-�T12H++2CO2��
�ʴ�Ϊ��2CH3OH+2H2O-12e-�T12H++2CO2��
��4��DΪ ����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ
����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ �������Ӿ۷�Ӧ������ʽΪ
�������Ӿ۷�Ӧ������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5��BΪ �����ӽṹ�к���
�����ӽṹ�к��� ������������ͬ���칹����
������������ͬ���칹���� 3�֣�
3�֣�
�ʴ�Ϊ��3�� ��
��
 ����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ
����E��R�������ȡ�����ʶ�λ������C+CH3OH=C11H14O3+H2O��֪��C�ķ���ʽӦΪC10H12O3�������к���1��-COOH��1��-OH�����E�Ľṹ��ʽ��֪CΪ ����BΪ
����BΪ ��AΪ
��AΪ ����DΪ
����DΪ ����
������1�������Ϸ�����֪��A���еĺ���������Ϊȩ��������ʽΪC10H11OCl���ʴ�Ϊ��ȩ����C10H11OCl��
��2��CΪ
 ��E�Ľṹ��ʽΪ
��E�Ľṹ��ʽΪ ��C����E�������۷�Ӧ��
��C����E�������۷�Ӧ���ʴ�Ϊ�����۷�Ӧ��
��3����֪��������R���л���R-OH����50%��˵��R-OHӦΪCH3OH���Ը�����Ϊȼ���ҵ������ҺΪ������Һ����õ�صĸ�����ӦΪ2CH3OH+2H2O-12e-�T12H++2CO2��
�ʴ�Ϊ��2CH3OH+2H2O-12e-�T12H++2CO2��
��4��DΪ
 ����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ
����Ũ���ᡢ���ȵ������·�Ӧ��ȥ��Ӧ����F��C11H12O2����FΪ �������Ӿ۷�Ӧ������ʽΪ
�������Ӿ۷�Ӧ������ʽΪ ��
���ʴ�Ϊ��
 ��
����5��BΪ
 �����ӽṹ�к���
�����ӽṹ�к��� ������������ͬ���칹����
������������ͬ���칹���� 3�֣�
3�֣��ʴ�Ϊ��3��
 ��
�����������⿼���л�����ƶϣ���Ŀ�ѶȽϴ�����Ҫע������Ŀ�е�һЩ������Ϣ�������EΪͻ�ƿڽ����ƶϣ�

��ϰ��ϵ�д�
�����Ŀ
 ���ʺ�������������Ӥ������ʯ��
���ʺ�������������Ӥ������ʯ��







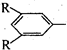

 ������R��R��������
������R��R��Ϊ������ �������� ����������ͬ���칹��Ľṹ��ʽ�� ���������������������� ��
�������� ����������ͬ���칹��Ľṹ��ʽ�� ���������������������� ��
 ����E��R�������ȡ�����ʶ�λ����֪R��R��Ϊ������
����E��R�������ȡ�����ʶ�λ����֪R��R��Ϊ������ ������������ͬ���칹���� �֣�д����������һ�ֵĽṹ��ʽ�� ��
������������ͬ���칹���� �֣�д����������һ�ֵĽṹ��ʽ�� ��