��Ŀ����
��A��B�������ʶ�����H��N��O��Na�е���������Ԫ����ɵ�ǿ����ʣ�A��ˮ��Һ�ʼ��ԣ�B��ˮ��Һ�����ԣ����ҳ�A��B���ܵ�������ϣ�Ҫ��A1����Һ��ˮ�ĵ���̶�С��A2��Һ��ˮ�ĵ���̶ȣ�B1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȡ�
��1��д����ѧʽA1________ B2_______��
��2����ͬ�¶��£���A1��B1�����ʵ���Ũ�����ʱ������Һ��ˮ�������H+�����ʵ���Ũ��֮��Ϊ_____________��
��3����B1��B2����Һ��pH = 5��������Һ��ˮ�����H+�����ʵ���Ũ��֮��Ϊ______��
�� 25��ʱ��Pt�缫���500mL amol��L CuSO4��Һ���Իش������й����⣺
��1��д�������ĵ缫��Ӧʽ�� ____________��
��2������C(Cu2+)Ϊ0.5a mol/Lʱ��ֹͣ��⣬��Ҫʹ��Һ�ָ�������ǰ��ͬ��״̬��������Һ�м���������____________��
A��CuSO4��ĩ B��CuSO4��5H2O C��CuO��ĩ D��Cu(OH)2
�٣�1��д����ѧʽA1:NaOH B2: NH4NO3
��2��1��1 ��3��10��4��1
��4OH�� ��4e��=O2��+2H2O, C
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

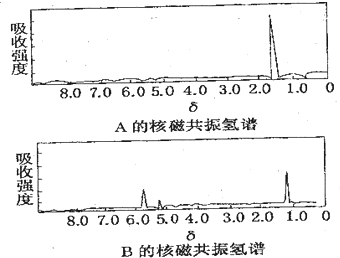 ��ͼ�ֱ���A��B�������ʵĺ˴Ź������ף���֪A��B�������ʶ������࣬������6����ԭ�ӣ��Ը����������ʵĺ˴Ź��������Ʋ�A��B�п���������ģ�������
��ͼ�ֱ���A��B�������ʵĺ˴Ź������ף���֪A��B�������ʶ������࣬������6����ԭ�ӣ��Ը����������ʵĺ˴Ź��������Ʋ�A��B�п���������ģ�������

