��Ŀ����
ij��Һ�У�ֻ���ܺ������������еļ��֣�
��ÿ��ȡ100.00mL��Һ����ʵ�飺�ٵ�һ�ݼ�����������Һ�г����������ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g������˵��������ǣ� ��
A��C�� ��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L
��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L
C�������ӿ��ܴ��� D��һ����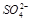 ��
��
| ������ | K+��Mg2��Fe3+��Al3+ |
| ������ |  �� ��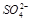 �� �� |
A��C��
 ��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L
��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L C�������ӿ��ܴ��� D��һ����
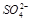 ��
��
C
����������ٵ�һ�ݼ�����������Һ�г�������,�˳�����һ��Ϊ�Ȼ�������Ϊ������̼����Ĵ��ڡ��ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g���˳���������̼�ᱵ�����ᱵ�Ļ�����������������ϴ�ӣ������ʣ��2.33g�������2.33g�ij��������ᱵ����ô6.27-2.33=3.94g������̼�ᱵ��������������0.02mol��C��
 ��="0.02mol��0.1L=0.2" mol/L����û��ȷ���Ƿ��������ӣ�����B��Dѡ�����ѡ��C��
��="0.02mol��0.1L=0.2" mol/L����û��ȷ���Ƿ��������ӣ�����B��Dѡ�����ѡ��C������������Ҫץס�����Ӳ�һ�����ھͿ��Խ�𣬹ؼ�����Ϥ����֮��ķ�Ӧ�����������⡣

��ϰ��ϵ�д�
 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
�����Ŀ