��Ŀ����
����Ŀ�����Ӻ˴Ź��������о��л�������ṹ�������ֶ�֮һ���ṹ�еĵ�Ч��ԭ�Ӻ˴Ź������ж���������Ӧ�ķ�ֵ(�ź�)�����з��ǿ����ṹ�е�Hԭ���������ȡ��Իش�
(1)�ṹ��ʽΪ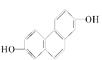 ���л�������ں˴Ź������й۲�������ǿ��֮��Ϊ___________��
���л�������ں˴Ź������й۲�������ǿ��֮��Ϊ___________��
(2)ʵ���пɸ��ݺ˹������Ϲ۲쵽Hԭ�Ӹ����ķ�ֵ�����ȷ���л�������Ľṹ������ʽΪC3H6O2����״�л�������˴Ź������Ϸ�������ȶ�ǿ�Ƚ������֣����Ƿֱ��Ǣ�3��3����3��2��1����3��1��1��1����1��2��2��1��
��ֱ��ƶϳ����Ӧ�Ľṹ��ʽ��
��________________________�� �� ___________________________��
��________________________�� �� ___________________________��
(3)���CH3CH2CH2CH3������˴Ź������Ͽɹ۲쵽���ַ壬��CH3CH=CHCH3�ĺ˴Ź�������4�ַ壬�����ռ�ṹ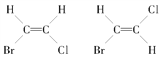 �����Ͳ���4�����ԭ��_______________��
�����Ͳ���4�����ԭ��_______________��
���𰸡� 1��1��1��1��1 CH3COOCH3 CH3CH2COOH��HCOOCH2CH3 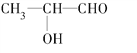 HO��CH2��CH2��CHO ��ΪCH3CH=CHCH3�����ֿռ�ṹ��
HO��CH2��CH2��CHO ��ΪCH3CH=CHCH3�����ֿռ�ṹ��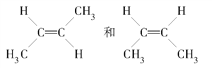 ÿ�ֽṹ�ں˴Ź������ϸ���2���壬���Թ�����4����
ÿ�ֽṹ�ں˴Ź������ϸ���2���壬���Թ�����4����
��������(1) 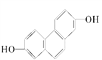 ����д�����£�
����д�����£� 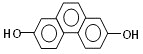 ���ṹ���ҶԳƣ�����������ԭ�ӣ���Ϊ2�����ں˴Ź������й۲�������ǿ��֮��Ϊ1�U1�U1�U1�U1��
���ṹ���ҶԳƣ�����������ԭ�ӣ���Ϊ2�����ں˴Ź������й۲�������ǿ��֮��Ϊ1�U1�U1�U1�U1��
(2) C3H6O2Ϊ��״�л�����������Ͷ�Ϊ1������Ϊһ��̼̼˫����һ���Ȼ���һ��ȩ�����ʻ����������Ϊ3:3ʱ��ֻ�������⣬�ṹ��ʽΪCH3COOCH3���������Ϊ3��2��1ʱ���������⣬�ṹ��ʽΪCH3CH2COOH��HCOOCH2CH3���������Ϊ3��1��1��1ʱ����4���⣬��ṹ��ʽΪ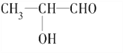 ���������Ϊ1��2��2��1ʱ�������⣬���ϵ�ֻ��HO��CH2��CH2��CHO��
���������Ϊ1��2��2��1ʱ�������⣬���ϵ�ֻ��HO��CH2��CH2��CHO��
(3) CH3CH=CHCH3�����ֿռ�ṹ����ʽ�ṹ��˳ʽ�ṹ��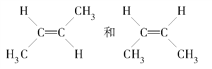 ��ÿ�ֽṹ�ں˴Ź������ϸ���2���壬���Թ�����4���塣
��ÿ�ֽṹ�ں˴Ź������ϸ���2���壬���Թ�����4���塣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���Ͼɵ���������л������ã�����ˮ�����������Ⱦ��ij��ȤС��ԷϾ��������������[��Ni(OH)2��̼�ۡ�Fe2O3��Ϳ�����������Ƴ�]���л����о������ʵ����������ͼ��ʾ��
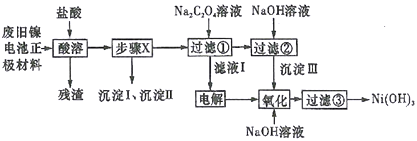
��֪����NiCl2������ˮ��Fe3+��������Ni2+,Cl2������Ni2+ΪNi3+;
��NiO+2HCl=NiCl2+H2O;
��Fe3+��Al3+��Ni3+������������ʽ����ʱ��Һ��pH���±���ʾ��
���� | Fe3+ | Al3+ | Ni2+ |
��ʼ����ʱ��pH | 1.5 | 3.5 | 7.0 |
��ȫ����ʱ��pH | 3.2 | 4.8 | 9.0 |
��Ksp[Ni(OH)2]=5.0��10-16��Ksp(NiC2O4)=4.0��10-10��
��ش��������⣺
��1�����ܺ�������������Ҫ�ɷ�__________(����������)��
��2������X�IJ���Ϊ��NiO������ҺpH�����������ij�������__________(�ѧʽ)�����ڹ�����pH���ܵ���___________��
��3����֪�ܽ�ȣ�NiC2O4>NiC2O4��H2O>NiC2O4��2H2O�������Na2C2O4��Һ�������ij�����__________��
��4��д�����ɳ����������ӷ���ʽ__________���÷�Ӧ��ƽ�ⳣ��Ϊ__________��
��5���û�ѧ��Ӧ����ʽ��ʾ������ת��ΪNi(OH)3________________________��
��6������������μ���Ni(OH)3��ϴ�Ӹɾ�____________________________��