��Ŀ����
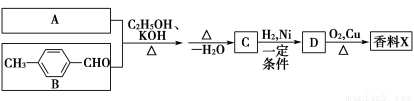
�����ؾ��п�ͻ���Ԥ�����������ã���ϳ�·�����£�
�����������������ºϳ�·��ã�

(1)�л������ķ���ʽΪ________�����й����ŵ�����Ϊ�Ѽ���________��
(2)�л�����������Ϊ________�����������Ļ�ѧ����ʽΪ(ע����Ӧ����)��_________________________________________________________________��
(3)д��һ�ַ�����������������ͬ���칹��Ľṹ��ʽ
_______________________________________________________��
�������ϵ�һ����ȡ������2��
��1 mol������ˮ�⣬�������3 mol NaOH
(4)��Ӧ���з�Ӧ���ԭ��������Ϊ100 %����д���÷�Ӧ�Ļ�ѧ����ʽ
____________________________________________________________��
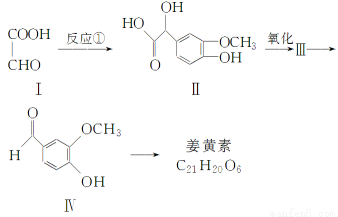
��(1)C8H8O3��(��)�ǻ���ȩ��
����������(1)�ڸ��л����й��������ֹ����ţ���O��Ϊ�Ѽ�����CHOΪȩ������OHΪ�ǻ���(2)��ת��ͼ�Ƴ�����1,2?�������飬��Ϊ�Ҷ�������Ϊ�Ҷ�ȩ�������������ķ�ӦΪ±�����ڼ��������µ�ˮ�ⷴӦ��
(3)�����������ϵ�һ����ȡ������2�֣��Ƴ������ϵ�ȡ�����ɶ�λȡ������������֪��ͬ���칹���к������Ľṹ����1 mol��ͨ����ˮ��ʱֻ������1 mol NaOH����������ˮ�⣬�������3 mol NaOH�����֪
��ͬ���칹���к���1�����ǻ��Լ�ˮ���������1�����ǻ����������ɴ˿����Ƴ���ͬ���칹��Ľṹ��

���ݱ�����Ϣ���ж�������������ȷ����(����)
��� | ������ | ��ԭ�� | ������Ӧ�� | �������� | ��ԭ���� |
�� | Cl2 | FeBr2 | / | Fe3����Br2 |
|
�� | KClO3 | Ũ���� | / | Cl2 |
|
�� | KMnO4 | H2O2 | H2SO4 | O2 | Mn2�� |
A.�ɱ����������Ϣ��֪ͨ��Cl2������ͬ������������ܲ�ͬ
B��������ǿ���ıȽϣ�KClO3>Fe3��>Cl2>Br2
C���������鷴Ӧ�Ļ�ԭ������KCl������ת����Ŀ��6e��
D���������鷴Ӧ�����ӷ���ʽΪ2MnO4����3H2O2��6H��=2Mn2����4O2����6H2O