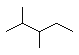
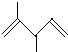
��Ŀ����
����Ŀ�����з����п���˵��2HI(g)![]() H2(g)+I2(g)�Ѵﵽƽ����ǣ��ٵ�λʱ��������n mol H2��ͬʱ����n mol HI����һ��H�CH�����ѵ�ͬʱ������H�CI�����ѣ��۰ٷ������(HI)=��(I2)���ܷ�Ӧ������(H2)=��(I2)=1/2��(HI)ʱ���� c(HI):c(H2):c(I2)=2:1:1ʱ�����¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯�����¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯��������һ������������ƽ����Է����������ٱ仯�����¶Ⱥ����һ��ʱ������������ɫ���ٱ仯�����¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯���� ��
H2(g)+I2(g)�Ѵﵽƽ����ǣ��ٵ�λʱ��������n mol H2��ͬʱ����n mol HI����һ��H�CH�����ѵ�ͬʱ������H�CI�����ѣ��۰ٷ������(HI)=��(I2)���ܷ�Ӧ������(H2)=��(I2)=1/2��(HI)ʱ���� c(HI):c(H2):c(I2)=2:1:1ʱ�����¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯�����¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯��������һ������������ƽ����Է����������ٱ仯�����¶Ⱥ����һ��ʱ������������ɫ���ٱ仯�����¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯���� ��
A. �ڢۢ� B. �٢ܢ� C. �ڢߢ� D. ����
���𰸡�C
��������
�����жϿ��淴Ӧ��ƽ��ı�־��һ�ǿ����淴Ӧ�����Ƿ���ȣ����ǿ������ʵ�Ũ���Ƿ�ı䣨�����ֵİٷֺ����Ƿ�ı䣩������ʾv������=v���棩��Ũ�Ȳ�������ɫ���ٱ仯��Ũ��һ�������ڸ÷�Ӧ��![]() �ķ�Ӧ�������������������仯���������ѹǿ��ƽ����Է����������ܶȾ�������Ϊ�ж�ƽ��״̬�ı�־����C��ȷ��
�ķ�Ӧ�������������������仯���������ѹǿ��ƽ����Է����������ܶȾ�������Ϊ�ж�ƽ��״̬�ı�־����C��ȷ��

����Ŀ��![]() ʱ����
ʱ����![]() ���ܱ������г���
���ܱ������г���![]() ��
��![]() ��һ�������·�����Ӧ��
��һ�������·�����Ӧ��![]() �����
�����![]() ��
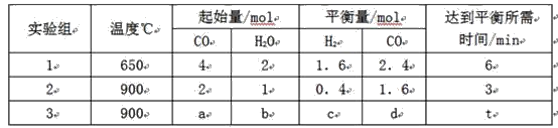
��![]() ��Ũ����ʱ��仯���±���ʾ������˵������ȷ����
��Ũ����ʱ��仯���±���ʾ������˵������ȷ����
ʱ�� |
|
|
|
| 6 | 0 | �� |
| 3 | 1 |
|
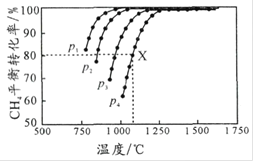
A.![]() ʱ����
ʱ����![]()
B.![]() ʱ���������¶Ȼ��ٳ���
ʱ���������¶Ȼ��ٳ���![]() ���壬���������
���壬���������![]() ��ת����
��ת����
C.![]() ʱ��
ʱ��![]() ��
��![]() ��
��![]()
D.![]() ʱ��ƽ�ⳣ��
ʱ��ƽ�ⳣ��![]() ��
��![]() ��
��![]() ��ת�������
��ת�������