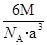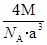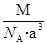��Ŀ����
�±����г���Ӧ���ʵ��۵㣬�й��ж���ȷ����
A��ֻҪ���н��������ӵľ����һ�������Ӿ���
B��AlF3��AlCl3�����ۻ�ʱ���˷�������������������ͬ
C��ͬ��Ԫ�ص������ﲻ�����γɲ�ͬ���͵ľ���
D������������۵㲻һ���ȷ��Ӿ����
| Na2O | Na | AlF3 | AlCl3 | Al2O3 | BCl3 | CO2 | SiO2 |
| 920 �� | 97.8 �� | 1291 �� | 190 �� | 2073 �� | ��107 �� | ��57 �� | 1723 �� |
B��AlF3��AlCl3�����ۻ�ʱ���˷�������������������ͬ
C��ͬ��Ԫ�ص������ﲻ�����γɲ�ͬ���͵ľ���
D������������۵㲻һ���ȷ��Ӿ����
D
������������������к��н��������ӣ�A����ȷ���Ȼ������۵�ͣ��γɵľ��������Ƿ��Ӿ��壬�ۻ��˷��������������Ƿ��Ӽ���������AlF3�γɵľ������������Ӿ��壬�˷��������Ӽ���B����ȷ��C��Si��ͬһ���壬������̼Ϊ���Ӿ��壬��������Ϊԭ�Ӿ��壬��C�����Ƶ��۵�����Ȼ������۵㣬���ǽ������壬����ѡ��D��ȷ����ѡD��
������������Ҫ�ǿ���ѧ�����������֪���ݷ��������ɺ��ܽ����������������ʱע������е����ݷ�����Ԫ�ص�λ�ã�����������ѧ�����ݷ��������������������������е��Ѷȵ����⡣

��ϰ��ϵ�д�
 С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
�����Ŀ

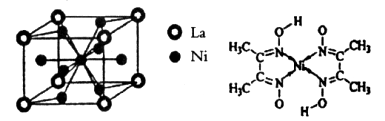
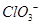 �Ŀռ乹��Ϊƽ��������
�Ŀռ乹��Ϊƽ��������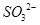 ������ԭ�Ӿ�Ϊsp3�ӻ�
������ԭ�Ӿ�Ϊsp3�ӻ�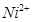 ��
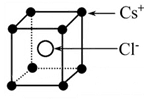
��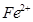 �����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO_________________FeO���������������
�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO_________________FeO���������������