��Ŀ����
2MnO2+Zn+2H2O=2MnOOH+Zn��OH��2����ҵ�������̿�Ϊԭ�ϣ��������������Ʊ��ߴ��ȶ������̵��������£����̿������н���Ԫ�������������¾�����������ʽ���ڣ���

ij���̿����Ҫ�ɷ�ΪMnO2������Si��16.27%����Fe��5.86%����Al��3.24%����Zn��2.68%����Cu��0.86%����Ԫ�صĻ��������������������������ʽ��ȫ����ʱ����Һ��pH�����������Ksp����Ϊ25��ʱ�����±����ش��������⣺
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mn��OH��2 | Cu��OH��2 | Zn��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 10.4 | 6.7 | 8.0 |
| ������ | CuS | ZnS | MnS | FeS | ||
| Ksp | 8.5��10-45 | 1.2��10-23 | 1.4��10-15 | 3.7��10-14 |
��2������A����Ҫ�ɷ�Ϊ
��3������MnS��Ŀ���dz�ȥ
��4������п�̵���У�MnO2����ĵ缫��ӦʽΪ
��5���ӷϾɼ���п�̵���пɻ������õ�������
��2������Ŀ��Ϣ��֪�������Ĺ���Һ�к���Mn2+��Fe3+��Al3+��Cu2+��Zn2+��Fe2+���������������������������ʽ��ȫ����ʱ��Һ��pH�����жϣ�
��3�����ݹ������̼������Ksp�жϣ�
��4���ɼ���п�̸ɵ�صĵ�ط�Ӧʽ��֪��Zn��������MnO2�õ���������������MnOOH���ɵ���غ��֪������OH-����Ԫ���غ��֪������ˮ���뷴Ӧ��
��5���Ӽ���п�̸ɵ�ص�ԭ���жϣ�
�ʴ�Ϊ��2FeSO4+MnO2+2H2SO4=MnSO4+Fe2��SO4��3+2H2O��
��2�������Ĺ���Һ�к���Mn2+��Fe3+��Al3+��Cu2+��Zn2+��Fe2+���Ӱ�ˮ��pH��5.4���������������������������ʽ��ȫ����ʱ��Һ��pH��֪��Fe3+��Al3+��ȫת��ΪFe��OH��3��Al��OH��3�������������Ӳ�������������A����Ҫ�ɷ�Ϊ
Fe��OH��3��Al��OH��3��
�ʴ�Ϊ��Fe��OH��3��Al��OH��3��
��3���������������Ksp��֪������MnS��Ϊ�������ܽ�ȸ�С��CuS��ZnS����ȥCu2+��Zn2+��
�ʴ�Ϊ��Cu2+��Zn2+��
��4������п�̸ɵ����Zn����������MnO2�������õ��ӣ���缫��ӦʽӦΪMnO2+H2O+e-=MnOOH+OH-��
�ʴ�Ϊ��MnO2+H2O+e-=MnOOH+OH-��
��5���Ӽ���п�̸ɵ�ص�ԭ�Ͽ�֪����Ͼɵ�ؿɻ������õ�����Ϊп��MnO2��
�ʴ�Ϊ��п���������̣�

 ��У����ϵ�д�
��У����ϵ�д�
ij���̿����Ҫ�ɷ�ΪMnO2��������Si(16.27%)��Fe(5.86%)��Al(3.42%)��Zn(2.68%)��Cu��0.86%����Ԫ�صĻ��������������������������������ʽ��ȫ����ʱ��Һ��pH���±����ش��������⣺
������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Cu(OH)2 | Zn(OH)2 | CuS | ZnS | MnS | |
pH | 5.2 | 3.2 | 9.7 | 10.4 | 6.7 | 8.0 | �ݨC0.42 | ��2.5 | ��7 | ��7 |
��1���������������������½�MnO2��ԭΪMnSO4�����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��
��2������A����Ҫ�ɷ��� ��
��3������MnS��Ŀ���dz�ȥ ���ʡ�
��4������п�̵���У�MnO2����ĵ缫��Ӧ����ʽΪ ��
��5���ӷϾɼ���п�̵���п��Ի������õ������� ��д���֣���
��������������п�̸ɵ�صĻ���ԭ�ϣ���ͨп�̸ɵ�صĵ�ط�ӦʽΪ��
2MnO2+Zn+2H2O=2MnOOH+Zn(OH)2����ҵ�������̿�Ϊԭ�ϣ��������������Ʊ��ߴ��ȶ������̵��������£����̿������н���Ԫ�������������¾�����������ʽ���ڣ���
ij���̿����Ҫ�ɷ�ΪMnO2������Si��16.27%����Fe��5.86%����Al��3.24%����Zn��2.68%����Cu��0.86%����Ԫ�صĻ��������������������������ʽ��ȫ����ʱ����Һ��pH�����������Ksp����Ϊ25��ʱ�����±����ش��������⣺
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 10.4 | 6.7 | 8.0 |
| ������ | CuS | ZnS | MnS | FeS |
|
|
| Ksp | 8.5��10��45 | 1.2��10��23 | 1.4��10��15 | 3.7��10��14 |
|
|
��1���������������������½�MnO2��ԭΪMnSO4�����ʱ��������Ҫ��ѧ����ʽΪ��
��
��2������A����Ҫ�ɷ�Ϊ ��
��3������MnS��Ŀ���dz�ȥ ���ʡ�
��4������п�̵���У�MnO2����ĵ缫��ӦʽΪ ��
��5���ӷϾɼ���п�̵���пɻ������õ������� ��д���֣���
��7�֣���������������п�̸ɵ�صĻ������ϣ���ҵ�������̿�Ϊԭ�ϣ��������������Ʊ��ߴ��������̵��������£�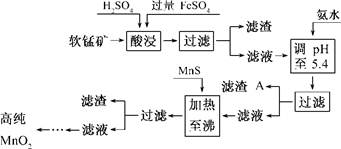
ij���̿����Ҫ�ɷ�ΪMnO2������Si��16��27%����Fe��5��86%����Al��3��42%����Zn��2��68%����Cu��0��86%����Ԫ�صĻ��������������������������������ʽ��ȫ����ʱ��Һ��pH���±����ش��������⣺
| ������ | pH |
| Al��OH��3 | 5��2 |
| Fe��OH��3 | 3��2 |
| Fe��OH��2 | 9��7 |
| Mn��OH��2 | 10��4 |
| Cu��OH��2 | 6��7 |
| Zn��OH��2 | 8��0 |
| CuS | �ݣ�0��42 |
| ZnS | ��2��5 |
| MnS | ��7 |
| FeS | ��7 |
��2������A����Ҫ�ɷ�Ϊ________��
��3������MnS��Ŀ���dz�ȥ________���ʣ�
��4������п�̸ɵ���У�MnO2����ĵ缫��Ӧ����ʽΪ__________________________��
��5���ӷϾɼ���п�̸ɵ���п��Ի������õ�������________��д�����֣���
