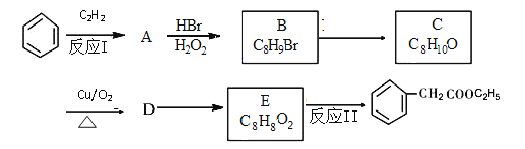
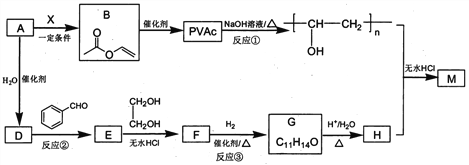
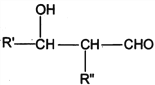
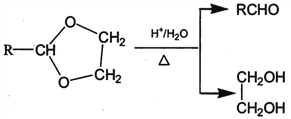
��Ŀ����
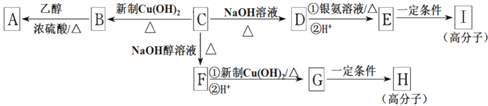
����Ŀ���������պ����ѧ����żȻ�з����׳�����ϣ��ܴ�����������ϵ��ʵ�飬֤ʵ�Ʒ۳�ij�������ɽ���۱���ϩ(PS)������ɽ������ϩ(PE)���۱���ϩ�ڳ泦�ڽ����ʾ��ͼ���£�����˵����ȷ����
A. �ڳ泦�����������£��۱���ϩ����̼̼˫��
B. �ڳ泦�����������£��۱���ϩֱ�ӽ���ΪCO2����
C. �ڳ泦�����������£��۱���ϩ�������⣬����������
D. ����ϩ��۱���ϩ��ͬϵ�����ϩ��������
���𰸡�C
�����������۱���ϩ�ṹ���Ѿ�������̼̼˫����������̼̼˫����A����������ͼʾ��֪���۱���ϩ�Ƚ���Ϊ�ͷ����м������ΪCO2���ӣ�B�������۱���ϩ�Ǹ߷��ӣ����������ΪС���ӵ����ʣ���������С��C��ȷ������ϩ��۱���ϩ���߽ṹ�����ƣ�����ͬϵ���ϵ���Ҷ������Ѿ�û��̼̼˫�������پ߱�ϩ�������ʣ�D��������ȷѡ��C��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ����ij��Br����ˮ����ȡBr2�Ĺ��̰��������ˡ���������ȡ����ѡ�������ȡ����������Ȳ��衣��֪��
���� | Br2 | CCl4 | ��ʮ���� |
�ܶ�/g��cm-3 | 3.119 | 1.595 | 0.753 |
�е�/�� | 58.76 | 76.8 | 215~217 |
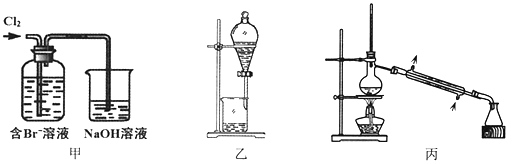
����˵������ȷ����
A. ��װ����Br�������ķ�ӦΪ��2Br-+ Cl2 = Br2 + 2Cl-
B. ��װ����NaOH��Һÿ����0.1mol Cl2��ת��0.1mol e��
C. ����װ�ý�����ȡ���ܽ�Br2���л������²�
D. �ñ�װ�ý����������ռ�������Br2