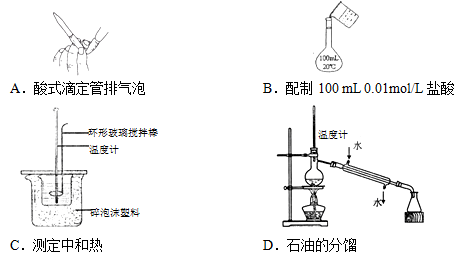
��Ŀ����
��8�֣���1����ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧʵ���ǻ�ѧѧϰ����Ҫ���ݡ����������յ�֪ʶ�жϣ�����ʵ������������У���ȷ���� ������ĸ��ţ���
E����ϡ����ε�pH��ֽ�ϣ��������ɫ���Ƚϣ��ⶨϡ�����pH
F����Һ������ʾ����Һ©�����²�Һ����¿ڷų����ϲ�Һ���Ͽڵ���
��2����ѧʵ���У�����ȷ�IJ������ʵ������ȷ�����һ����Ӱ�죬���á�>������<����=����д���пհף�
����������ƽ��ȡ8.4g�Ȼ��ƣ�����������Ȼ��Ƶ�λ�÷ŵߵ��ˣ�����ȡ�Ȼ��Ƶ����� 8.4g��
��������ƿ����500mL0.1mol��L-1NaOH��Һ������ʱ���ӿ̶��ߣ�����NaOH��Һ�����ʵ���Ũ�� 0.1mol��L-1��
���к͵ζ�ʵ��ʱ��ƿ������ˮϴ����δ�ñ�Һ��ϴ��������ƿ�д���Һ��Ũ�� ʵ��Ũ�ȡ�
| A�����Լ�ƿ��ȡ����ҩƷ������ʣ���ٷŻ�ԭ�Լ�ƿ |
| B������һ��������ͬ����ֽ����ƽ���������ϣ���NaOH�����������ֽ�ϳ��� |
| C���������ʱ�����ܽ���ˮ�ķ������Ͽڽ����¿ڳ� |
| D��NaOH��Һ���ܱ����ڴ��в��������Լ�ƿ�� |
F����Һ������ʾ����Һ©�����²�Һ����¿ڷų����ϲ�Һ���Ͽڵ���
��2����ѧʵ���У�����ȷ�IJ������ʵ������ȷ�����һ����Ӱ�죬���á�>������<����=����д���пհף�
����������ƽ��ȡ8.4g�Ȼ��ƣ�����������Ȼ��Ƶ�λ�÷ŵߵ��ˣ�����ȡ�Ȼ��Ƶ����� 8.4g��
��������ƿ����500mL0.1mol��L-1NaOH��Һ������ʱ���ӿ̶��ߣ�����NaOH��Һ�����ʵ���Ũ�� 0.1mol��L-1��
���к͵ζ�ʵ��ʱ��ƿ������ˮϴ����δ�ñ�Һ��ϴ��������ƿ�д���Һ��Ũ�� ʵ��Ũ�ȡ�
��ÿ��2�֣���8�֣�
��1��DF ��2���٣� �� �� �� ��
��1��DF ��2���٣� �� �� �� ��
��1��A����ȡ����ҩƷ������Ⱦ�����ܷŻ�ԭƿ��Na��K���⣩��B��NaOH�׳��⣬Ӧ��С�ձ�������C������ˮ���¿ڽ����Ͽڳ��������������෴���ﵽ�����Ƚ���Ч����D����ȷ�������������Ʒ�Ӧ�����ɹ����ƣ�������ԣ�E������Һ��pH�����õ��Dz�������F����ȷ��
��2��������=����+���룬����ŷ���ʵ������ֻ��7.6g��1g���������룩���ڶ��������ӣ����ƫ��Ũ��ƫС��������ƿ�м�����ˮ����ʵ����Ӱ�졣
��2��������=����+���룬����ŷ���ʵ������ֻ��7.6g��1g���������룩���ڶ��������ӣ����ƫ��Ũ��ƫС��������ƿ�м�����ˮ����ʵ����Ӱ�졣

��ϰ��ϵ�д�
 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
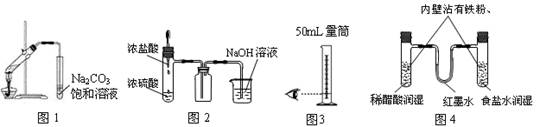
 ����ʵ�������ȷ���ǣ� ��
����ʵ�������ȷ���ǣ� ��