��Ŀ����
̼���⡢��Ԫ�ص�������Ϊ6��1��8���л���A��B��C�������Ƕ��ܷ���������Ӧ���������ܷ���ˮ�ⷴӦ��B1��B2��B��ͬ���칹�塣��֪��
A�ڳ�����Ϊ���壬A+C6H5OH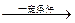 Z(�߷��ӻ�����)
Z(�߷��ӻ�����)
B1����Ϊ��״���壺B1+Na2CO3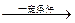 X+CO2+H2O��X+NaOH
X+CO2+H2O��X+NaOH 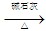 Y(�����)
Y(�����)
B2Ϊ��ɫҺ�壬Ҳ�ܷ���������Ӧ��1 mol C��ȫȼ����Ҫ3 mol������
?CH(OH)2 �� ?C(OH)2? ���ȶ�������ˮ�γ�?CH=O�� ?CO?���Իش�
��1��A��Z�ķ�Ӧ����
��2��A��B�Ľṹ��ʽ��A ��B ��
��3��д��B2���������Ʒ�Ӧ�Ļ�ѧ����ʽ�� ��
��4��C�Ľṹ��ʽ ����C��Ϊͬ���칹�� �������������������
�������������������
�Ľṹ��ʽ �� ��
A�ڳ�����Ϊ���壬A+C6H5OH
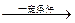 Z(�߷��ӻ�����)
Z(�߷��ӻ�����)B1����Ϊ��״���壺B1+Na2CO3
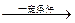 X+CO2+H2O��X+NaOH
X+CO2+H2O��X+NaOH 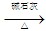 Y(�����)
Y(�����)B2Ϊ��ɫҺ�壬Ҳ�ܷ���������Ӧ��1 mol C��ȫȼ����Ҫ3 mol������
?CH(OH)2 �� ?C(OH)2? ���ȶ�������ˮ�γ�?CH=O�� ?CO?���Իش�
��1��A��Z�ķ�Ӧ����
��2��A��B�Ľṹ��ʽ��A ��B ��
��3��д��B2���������Ʒ�Ӧ�Ļ�ѧ����ʽ�� ��
��4��C�Ľṹ��ʽ ����C��Ϊͬ���칹��
 �������������������
��������������������Ľṹ��ʽ �� ��
��8�֣�(1)��ȥ��Ӧ��1�֣�
(2) HCHO��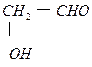 ��2�֣�
��2�֣�
(3) HCOOCH3+NaOH(H2O) HCOONa+CH3OH��2�֣�
HCOONa+CH3OH��2�֣�
(4)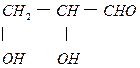 ��1�֣�
��1�֣�
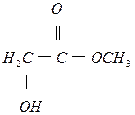
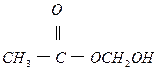 ��2�֣�
��2�֣�
(2) HCHO��
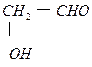 ��2�֣�
��2�֣�(3) HCOOCH3+NaOH(H2O)
 HCOONa+CH3OH��2�֣�
HCOONa+CH3OH��2�֣�(4)
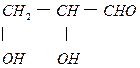 ��1�֣�
��1�֣�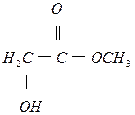
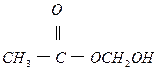 ��2�֣�
��2�֣���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
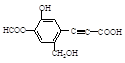 ������˵����ȷ����
������˵����ȷ����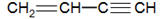 ����������̼ԭ�ӿ��ܴ���ͬһֱ����
����������̼ԭ�ӿ��ܴ���ͬһֱ����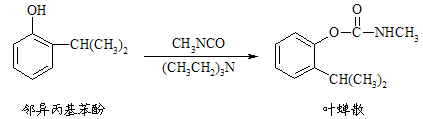
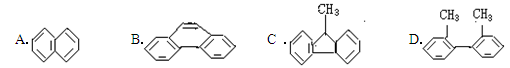
 �ǻ��ϵ�������ã��Խ�ǿ������
�ǻ��ϵ�������ã��Խ�ǿ������