��Ŀ����
���淴ӦN2��g��+3H2(g)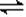 2NH3(g)�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
2NH3(g)�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
�ٵ�λʱ��������n mol N2��ͬʱ������3n mol H2
�ڶϿ�1mol H-H����ͬʱ�Ͽ�2 molN-H��
����N2��H2��NH3��Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ1:3:2
�ܻ�������ѹǿ���ٸı�
�ݻ��������ܶȲ��ٸı�
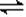 2NH3(g)�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
2NH3(g)�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ���ٵ�λʱ��������n mol N2��ͬʱ������3n mol H2
�ڶϿ�1mol H-H����ͬʱ�Ͽ�2 molN-H��
����N2��H2��NH3��Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ1:3:2
�ܻ�������ѹǿ���ٸı�
�ݻ��������ܶȲ��ٸı�
| A���ڢܡ����� | B���٢� | C���٢ۢ� | D���ڢۢܢ� |
A
�����������ѧ��Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��䣬�ɴ�������һЩ���������䡣
��ͬλ�淴Ӧ���ʣ��ʴ����������Ƿ�ﵽƽ��״̬����ѧ��Ӧ����֮�ȶ����ڻ�ѧ������֮�ȣ��ʴ����ݷ�Ӧǰ��������������䣬������������䣬�����Ƿ�ﵽƽ��״̬��������ܶȶ����䣬������Ϊ�ж��Ƿ�ﵽƽ��״̬�����ݣ��ʴ���
��ѡA��
���������⿼�鿼�黯ѧƽ��״̬���жϣ���Ŀ�Ѷ��еȣ�����ע�����淴Ӧ���ʹ�ϵ��ȷ����Ϊ�״��㣬����ʱע����ջ�ѧ����ʽ��������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
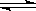 2NH3���ԣ������������Ʒ�Ӧ����1mol N2��3mol H2��Ӧ����������2mol NH3
2NH3���ԣ������������Ʒ�Ӧ����1mol N2��3mol H2��Ӧ����������2mol NH3 2NH3(g) ��H<0�����¶Ȳ��䣬���ƽ��ʱ���й��������£�
2NH3(g) ��H<0�����¶Ȳ��䣬���ƽ��ʱ���й��������£�
 CO2 (g)+ Fe(s)��H��0����ƽ�ⳣ���ɱ�ʾΪK=c(CO2)/c(CO)����֪1100��ʱ��K=0.263��
CO2 (g)+ Fe(s)��H��0����ƽ�ⳣ���ɱ�ʾΪK=c(CO2)/c(CO)����֪1100��ʱ��K=0.263�� ��CO�������____________ (ѡ���������С�����䡱)��ƽ�ⳣ��Kֵ_____________(ѡ���������С�����䡱)��
��CO�������____________ (ѡ���������С�����䡱)��ƽ�ⳣ��Kֵ_____________(ѡ���������С�����䡱)�� ��
�� ����ʱ����Ӧ�Ƿ��ڻ�ѧƽ��״̬__________ (ѡ��ǡ���)����ѧ��Ӧ�ٶ�
����ʱ����Ӧ�Ƿ��ڻ�ѧƽ��״̬__________ (ѡ��ǡ���)����ѧ��Ӧ�ٶ� (��)__________
(��)__________ x C(g)���ڲ�ͬ�¶ȼ�ѹǿ��p1�� p2�������£���Ӧ��A��ת������ͼ��ʾ�������ж���ȷ����
x C(g)���ڲ�ͬ�¶ȼ�ѹǿ��p1�� p2�������£���Ӧ��A��ת������ͼ��ʾ�������ж���ȷ���� 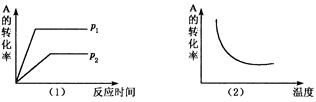
 2SO3(g)������ͼ����ȷ����(M��ʾ��������ƽ����Է�������)
2SO3(g)������ͼ����ȷ����(M��ʾ��������ƽ����Է�������)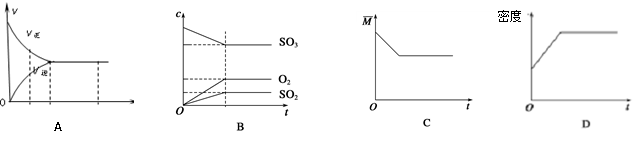
 cZ(g)+dW(g)����Ӧ�ﵽƽ������¶Ȳ��䣬������ѹ����ԭ����1/2��������ٴδﵽƽ��ʱ��W��Ũ��Ϊԭƽ���1.8������������������ȷ����
cZ(g)+dW(g)����Ӧ�ﵽƽ������¶Ȳ��䣬������ѹ����ԭ����1/2��������ٴδﵽƽ��ʱ��W��Ũ��Ϊԭƽ���1.8������������������ȷ���� CH3OH(g)
CH3OH(g) 