��Ŀ����
��Ȳ��C2H2�����л��ϳɹ�ҵ��һ��ԭ�ϡ���ҵ������CaC2��ˮ��Ӧ������Ȳ��
(1)CaC2��C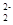 ��O
��O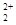 ��Ϊ�ȵ����壬�ȵ�����ṹ���ƣ���1 ��O
��Ϊ�ȵ����壬�ȵ�����ṹ���ƣ���1 ��O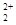 �к��еĦм���ĿΪ ��
�к��еĦм���ĿΪ ��
(2)����Ȳͨ��[Cu(NH3)2]Cl��Һ����Cu2C2����ɫ������Cu����̬��������Ų�ʽΪ
��
(3)��Ȳ�������ᷴӦ�ɵñ�ϩ��( H2C��CH��C��N )����ϩ�������̼ԭ�ӹ���ӻ������� �������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ________��
(1)CaC2��C
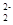 ��O
��O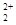 ��Ϊ�ȵ����壬�ȵ�����ṹ���ƣ���1 ��O
��Ϊ�ȵ����壬�ȵ�����ṹ���ƣ���1 ��O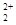 �к��еĦм���ĿΪ ��
�к��еĦм���ĿΪ ��(2)����Ȳͨ��[Cu(NH3)2]Cl��Һ����Cu2C2����ɫ������Cu����̬��������Ų�ʽΪ
��
(3)��Ȳ�������ᷴӦ�ɵñ�ϩ��( H2C��CH��C��N )����ϩ�������̼ԭ�ӹ���ӻ������� �������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ________��
(1) 2��2�֣�(2)1s22s22p63s23p63d10��2�֣� (3)sp1�ӻ���sp2�ӻ�����2�֣��� 3��2�֣�
�����������1��C
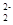 �к���̼̼����������2���м�������C
�к���̼̼����������2���м�������C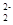 ��O
��O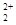 ��Ϊ�ȵ����壬�ȵ�����ṹ���ƣ���1 ��O
��Ϊ�ȵ����壬�ȵ�����ṹ���ƣ���1 ��O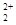 �к��еĦм���ĿΪ2����
�к��еĦм���ĿΪ2������2�����ݹ���ԭ����֪��Cu����̬��������Ų�ʽΪ1s22s22p63s23p63d10��
��3��̼̼˫����ƽ���ͽṹ��̼��������ֱ���ͽṹ�����ϩ�������̼ԭ�ӹ���ӻ�������sp2�ӻ���Ҳ��sp�ӻ��������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ3����
�������������е��Ѷȵ����⣬���������ǿ����Ҫ�ǶԻ���֪ʶ�Ĺ��̺ͼ��顣����Ĺؼ�����ȷ�йظ���ĺ��塢�ж����ݣ�Ȼ��������������ü��ɡ�����������ѧ���������������ͳ���˼ά������Ҳ�����ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ��֪����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
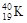 ��
��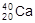 D��H��D��T
D��H��D��T