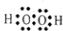��Ŀ����
��2009��3���𣬴�ī���硢�����ȹ�����ɢ��ȫ����ļ���H1N1�����б������飬����ȫ���ע�ͻ���Ӧ�ԣ�����ר�ұ�ʾ���������������������ǿ��������������Ԥ������H1N1�����У�
��1����̼���� ����ѧʽ2Na2CO3?3H2O2�� �׳ƹ���˫��ˮ����ɫ�ᾧ��������̼��������ˮ������ʱ���ֽ�����̼���ƺ������⣬�ǺܺõĹ������ͷż���
������д����������ĵ���ʽ______��������Ԫ�صĻ��ϼ���______��
��H2O2��ʱ����Ϊ��ҵ�������������С���ɫ�������������ƣ��������ɿ�ҵ��Һ�е��軯���KCN���������·�Ӧʵ�֣�
KCN+H2O2+H2O=A+NH3������������A�Ļ�ѧʽΪ______��H2O2����Ϊ����ɫ����������������______��
��ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�O2��MnO4-��H2O��Mn2+��H2O2��H+����֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2��O2��д���÷�Ӧ�����ӷ���ʽ______��
��ʵ������O2��Ҫ��4�ַ������û�ѧ����ʽд������һ��______��
��2��̼����ˮ��Һ�Լ��ԣ������ӷ���ʽ������ԭ��______��pHֵ��ͬ�Ģ�̼������Һ���ڴ�������Һ��������������Һ����Ũ���ɴ�С��˳���ǣ�______����д��ţ���
��3��Ư���������� ��NaClO2�� �ڳ��ºڰ����ɱ���һ�꣮������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��
HClO2��ClO2��+H++Cl-+H2O��δ��ƽ�����ڸ÷�Ӧ�У�����1molClO2����ʱ��ת�Ƶĵ�������______����
��4��ClO2���ȶ�������NaOH��Һ��H2O2��Ӧ��ת��Ϊ���ȶ����������� ��NaClO2�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
�⣺��1����H�����1�����ӣ�Oԭ�������6�����ӣ����������ĵ���ʽΪ ��H2O2��HΪ+1�ۣ��ɻ��������������ϼ۵Ĵ�����Ϊ0������Ԫ�صĻ��ϼ�Ϊ-1�ۣ�
��H2O2��HΪ+1�ۣ��ɻ��������������ϼ۵Ĵ�����Ϊ0������Ԫ�صĻ��ϼ�Ϊ-1�ۣ�
�ʴ�Ϊ�� ��-1��
��-1��
���ɷ�ӦKCN+H2O2+H2O=A+NH3�������������غ㶨�ɿ�֪��Ԫ���غ㡢ԭ���غ㣬��A�Ļ�ѧʽΪKHCO3��H2O2����Ϊ����ɫ������������Ԫ�صĻ��ϼ۽��ͣ������Ϊˮ��
������Ի�������Ⱦ���ʴ�Ϊ��KHCO3���ڷ�Ӧ��ϵ�в��������ʣ���Ի�������Ⱦ����
��MnO4-���������ԣ�H2O2���л�ԭ�ԣ�H2O2ֻ���������¹��̣�H2O2��O2����ԭ��ӦΪMnO4-��Mn2+�����������غ㶨�ɼ�����غ��֪�����ӷ�ӦΪ
2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O���ʴ�Ϊ��2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O��
�ܹ�������ֽ��������������÷�ӦΪ2H2O2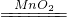 2H2O+O2�����ʴ�Ϊ��2H2O2
2H2O+O2�����ʴ�Ϊ��2H2O2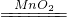 2H2O+O2����
2H2O+O2����
��2��̼�������ˮ������̼��������Ӻ����������ӣ����ӷ�ӦΪCO32-+H2O?HCO3-+OH-�������е�������Ӷ�Ӧ����Խ����ˮ��̶�Խ����pHԽ����Խǿ����Һ��pHԽ����pHֵ��ͬ�Ģ�̼������Һ���ڴ�������Һ��������������Һ���۵�Ũ����С��̼�������С�ڴ��ᣬ��ڵ�Ũ����ʴ�Ϊ���ڣ��٣��ۣ�
��3����HClO2��ClO2��+H++Cl-+H2O��֪����Ӧ����ClԪ�صĻ��ϼۼ������ֽ��ͣ�����1molClO2����ʱ��ת�Ƶĵ�����Ϊ1mol����4-3����NAmol-1=NA���ʴ�Ϊ��NA��
��4����Ӧ��ΪClO2��NaOH��H2O2��������ΪNaClO2����������ԭ��Ӧ�������غ��֪����ӦΪ2ClO2+2NaOH+H2O2�T2NaClO2+O2��+2H2O��
�ʴ�Ϊ��2ClO2+2NaOH+H2O2�T2NaClO2+O2��+2H2O��
��������1���������������ӷ�������ʽ������HΪ+1�ۼ����������������ϼ۵Ĵ�����Ϊ0��������
�ڸ��������غ㶨��������A�Ļ�ѧʽ����������������Ԫ�صĻ��ϼ۱仯��������
������������ԭ��Ӧ��������
�ܹ�������ֽ�������������
��2��̼�������ˮ������̼��������Ӻ����������ӣ����е�������Ӷ�Ӧ����Խ����ˮ��̶�Խ����pHԽ����Խǿ����Һ��pHԽ��
��3�����ݻ��ϼ۱仯������ת�Ƶĵ�������
��4�����÷�Ӧ��������������д��ѧ��Ӧ����ʽ��
���������⿼��������ԭ��Ӧ�����ӷ�Ӧ��ˮ���֪ʶ������֪ʶ��Ϊ�ۺϣ���ע���˶Ը߿�����Ŀ��鼰��������Ϣ�����黯ѧ֪ʶ����һ��������ϰ�⣮
 ��H2O2��HΪ+1�ۣ��ɻ��������������ϼ۵Ĵ�����Ϊ0������Ԫ�صĻ��ϼ�Ϊ-1�ۣ�
��H2O2��HΪ+1�ۣ��ɻ��������������ϼ۵Ĵ�����Ϊ0������Ԫ�صĻ��ϼ�Ϊ-1�ۣ��ʴ�Ϊ��
 ��-1��
��-1�����ɷ�ӦKCN+H2O2+H2O=A+NH3�������������غ㶨�ɿ�֪��Ԫ���غ㡢ԭ���غ㣬��A�Ļ�ѧʽΪKHCO3��H2O2����Ϊ����ɫ������������Ԫ�صĻ��ϼ۽��ͣ������Ϊˮ��
������Ի�������Ⱦ���ʴ�Ϊ��KHCO3���ڷ�Ӧ��ϵ�в��������ʣ���Ի�������Ⱦ����
��MnO4-���������ԣ�H2O2���л�ԭ�ԣ�H2O2ֻ���������¹��̣�H2O2��O2����ԭ��ӦΪMnO4-��Mn2+�����������غ㶨�ɼ�����غ��֪�����ӷ�ӦΪ
2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O���ʴ�Ϊ��2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O��
�ܹ�������ֽ��������������÷�ӦΪ2H2O2
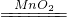 2H2O+O2�����ʴ�Ϊ��2H2O2
2H2O+O2�����ʴ�Ϊ��2H2O2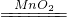 2H2O+O2����
2H2O+O2������2��̼�������ˮ������̼��������Ӻ����������ӣ����ӷ�ӦΪCO32-+H2O?HCO3-+OH-�������е�������Ӷ�Ӧ����Խ����ˮ��̶�Խ����pHԽ����Խǿ����Һ��pHԽ����pHֵ��ͬ�Ģ�̼������Һ���ڴ�������Һ��������������Һ���۵�Ũ����С��̼�������С�ڴ��ᣬ��ڵ�Ũ����ʴ�Ϊ���ڣ��٣��ۣ�
��3����HClO2��ClO2��+H++Cl-+H2O��֪����Ӧ����ClԪ�صĻ��ϼۼ������ֽ��ͣ�����1molClO2����ʱ��ת�Ƶĵ�����Ϊ1mol����4-3����NAmol-1=NA���ʴ�Ϊ��NA��
��4����Ӧ��ΪClO2��NaOH��H2O2��������ΪNaClO2����������ԭ��Ӧ�������غ��֪����ӦΪ2ClO2+2NaOH+H2O2�T2NaClO2+O2��+2H2O��
�ʴ�Ϊ��2ClO2+2NaOH+H2O2�T2NaClO2+O2��+2H2O��
��������1���������������ӷ�������ʽ������HΪ+1�ۼ����������������ϼ۵Ĵ�����Ϊ0��������
�ڸ��������غ㶨��������A�Ļ�ѧʽ����������������Ԫ�صĻ��ϼ۱仯��������
������������ԭ��Ӧ��������
�ܹ�������ֽ�������������
��2��̼�������ˮ������̼��������Ӻ����������ӣ����е�������Ӷ�Ӧ����Խ����ˮ��̶�Խ����pHԽ����Խǿ����Һ��pHԽ��
��3�����ݻ��ϼ۱仯������ת�Ƶĵ�������
��4�����÷�Ӧ��������������д��ѧ��Ӧ����ʽ��
���������⿼��������ԭ��Ӧ�����ӷ�Ӧ��ˮ���֪ʶ������֪ʶ��Ϊ�ۺϣ���ע���˶Ը߿�����Ŀ��鼰��������Ϣ�����黯ѧ֪ʶ����һ��������ϰ�⣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ