��Ŀ����
ŷ�����о�������1995��9��10�����Ƴ������ϵ�һ����ԭ�ӡ�����9������ԭ�ӣ��ҿ���������ȡ�����÷����ʵ���ƪ�¡�������������⣺
(1)����ԭ�ӵĽṹʾ��ͼ�У���ȷ����( )
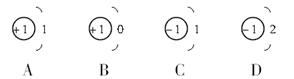
(2)�����ȡ�˷���ԭ�ӣ�������˵���У���ȷ����( )
A��������8������������ӣ�������8��������ĵ���
B��������8��������ĵ��ӣ�������8�������������
C��������8������������ӣ�������8�������������
D��������8������������ӣ�������8��������ĵ���
(3)���±�ʾ����������кͷ�Ӧ��ͨʽ��( )
A��H++OH+ H2O B��H++OH+
H2O B��H++OH+ H2O
H2O
C��H-+OH- H2O D��H++OH-
H2O D��H++OH- H2O
H2O
(1)����ԭ�ӵĽṹʾ��ͼ�У���ȷ����( )
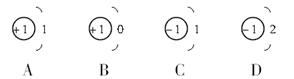
(2)�����ȡ�˷���ԭ�ӣ�������˵���У���ȷ����( )
A��������8������������ӣ�������8��������ĵ���
B��������8��������ĵ��ӣ�������8�������������
C��������8������������ӣ�������8�������������
D��������8������������ӣ�������8��������ĵ���
(3)���±�ʾ����������кͷ�Ӧ��ͨʽ��( )
A��H++OH+
 H2O B��H++OH+
H2O B��H++OH+ H2O
H2OC��H-+OH-
 H2O D��H++OH-
H2O D��H++OH- H2O
H2O ��1��C��2��D��3��A
��������ָ��������ͬ�������෴�����ӣ������Ӵ�����ɣ����Ӵ�����ɡ�

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ