��Ŀ����
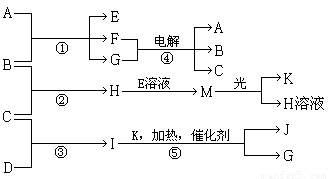
����A��E��F����ɫ��Ӧ�ʻ�ɫ������I�ķ�����4��ԭ����ɲ�������10�����ӣ�B��C��D��K�ڳ����¶������嵥�ʣ�G�ڳ���������ɫҺ�壬��Ӧ�١��ݶ������ڹ�ҵ�����ķ�Ӧ�����й�����֮������Ӧת����ϵ��ͼ��ʾ��
����д���пհף�
��1��д���������ʵĻ�ѧʽ��B��______��J��______��
��2��д�����з�Ӧ�ķ���ʽ���ٷ�Ӧ�ٵ����ӷ���ʽ��______���ڷ�Ӧ�ݵĻ�ѧ����ʽ��______��
��3����ͨ��״���£���1g C������B������ȼ������H����ʱ�ų�92.3kJ��������2mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ��______��

����д���пհף�
��1��д���������ʵĻ�ѧʽ��B��______��J��______��
��2��д�����з�Ӧ�ķ���ʽ���ٷ�Ӧ�ٵ����ӷ���ʽ��______���ڷ�Ӧ�ݵĻ�ѧ����ʽ��______��
��3����ͨ��״���£���1g C������B������ȼ������H����ʱ�ų�92.3kJ��������2mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ��______��
I�ķ�����4��ԭ����ɲ�������10�����ӣ�ӦΪNH3��G�ڳ���������ɫҺ�壬ӦΪH2O����ת����ϵ��֪��Ӧ��ӦΪ�����Ĵ���������KΪO��JΪNO����CΪH2��DΪN2������A��E��F����ɫ��Ӧ�ʻ�ɫ��Ӧ��������Ԫ�أ�M�ڹ��������¿�����O2��ӦΪHClO����HΪHCl��BΪCl2��EΪNaClO��AΪNaOH��FΪNaCl��
��1�������Ϸ�����֪BΪCl2��JΪNO���ʴ�Ϊ��Cl2��NO��
��2���ٷ�Ӧ��Ϊ�������������Ƶķ�Ӧ����Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
�ڷ�Ӧ��Ϊ�����Ĵ�������Ӧ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��3����1g H2������Cl2������ȼ������H����ʱ�ų�92.3kJ��������1molH2ȼ�շų�184.6kJ����������2HCl��g��=H2��g��+Cl2��g������H=+184.6 kJ?mol-1��
�ʴ�Ϊ��2HCl��g��=H2��g��+Cl2��g������H=+184.6 kJ?mol-1��
��1�������Ϸ�����֪BΪCl2��JΪNO���ʴ�Ϊ��Cl2��NO��
��2���ٷ�Ӧ��Ϊ�������������Ƶķ�Ӧ����Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
�ڷ�Ӧ��Ϊ�����Ĵ�������Ӧ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| �� |
��3����1g H2������Cl2������ȼ������H����ʱ�ų�92.3kJ��������1molH2ȼ�շų�184.6kJ����������2HCl��g��=H2��g��+Cl2��g������H=+184.6 kJ?mol-1��
�ʴ�Ϊ��2HCl��g��=H2��g��+Cl2��g������H=+184.6 kJ?mol-1��

��ϰ��ϵ�д�
�����Ŀ

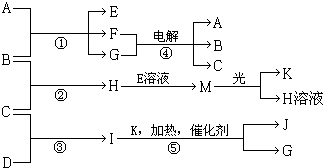 ����A��E��F����ɫ��Ӧ�ʻ�ɫ������I��1��������4��ԭ����ɲ�������10�����ӣ�B��C��D��K�ڳ����¶������嵥�ʣ�G�ڳ���������ɫҺ�壬��Ӧ�١��ݶ������ڹ�ҵ�����ķ�Ӧ�����й�����֮������Ӧת����ϵ��ͼ��ʾ�����ַ�Ӧ��������ȥ����
����A��E��F����ɫ��Ӧ�ʻ�ɫ������I��1��������4��ԭ����ɲ�������10�����ӣ�B��C��D��K�ڳ����¶������嵥�ʣ�G�ڳ���������ɫҺ�壬��Ӧ�١��ݶ������ڹ�ҵ�����ķ�Ӧ�����й�����֮������Ӧת����ϵ��ͼ��ʾ�����ַ�Ӧ��������ȥ����