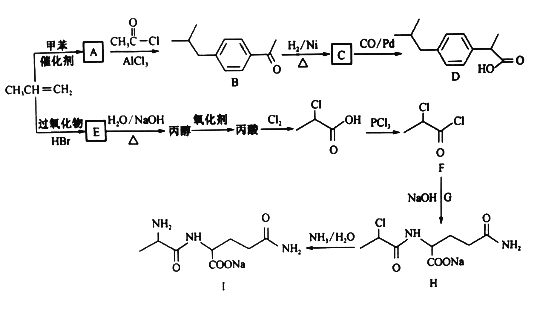
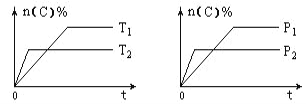
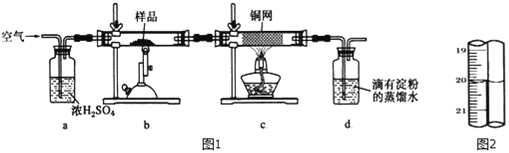
��Ŀ����
����Ŀ����ͼ�г��ˢ١������Ԫ�������ڱ��е�λ�ã� 
�밴Ҫ��ش��������⣮
��1��Ԫ�آ�������� �� Ԫ�آ������ڱ�������λ�� �� Ԫ�آܵ��⻯��е���ڢߵ��⻯��е㣬ԭ���� ��
��2���ܢޢߵ��⻯�ﰴ�ȶ���������ǿ��˳������д�⻯��Ļ�ѧʽ����
��3��Ԫ�آڢ��γ�ԭ�Ӹ�����Ϊ1��2�Ļ�����ĵ���ʽ�� ��
��4��Ԫ�آ��γɵ�һ���⻯���Ϊ������ͨʽ�� �� �����������ʽ��������ͬ���칹�壬�ṹ��ʽ�ֱ��� ��
��5���õ���ʽ��ʾ������γɻ�����Ĺ��� ��
���𰸡�
��1���ʣ��ڶ�����0�壻H2O���Ӽ����γ����
��2��PH3��H2S��H2O
��3��![]()
��4��CnH2n+2��C4H10��CH3CH2CH2CH3��CH3CH��CH3��CH3
��5��![]()
���������⣺��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪP����ΪS����ΪNe����ΪCl��
��1.������������ʣ�λ�ڵڶ�����0�壬Ԫ�آ�Ϊ����Ԫ�آܵ��⻯��е���ڢߵ��⻯��е㣬ԭ����H2O���Ӽ����γ���������Դ��ǣ��ʣ��ڶ�����0�壻H2O���Ӽ����γ������
��2.��ͬ����������ҷǽ�������ǿ��ͬ�������϶��·ǽ����Լ������ʷǽ�����O��S��P���ǽ�����Խǿ���⻯��Խ�ȶ������⻯���ȶ��ԣ�PH3��H2S��H2O�����Դ��ǣ�PH3��H2S��H2O��
��3.��Ԫ�آڢ��γ�ԭ�Ӹ�����Ϊ1��2�Ļ�����ΪCO2 �� �����ʽΪ ![]() �����Դ��ǣ�
�����Դ��ǣ� ![]() ��
��
��4.��Ԫ�آ��γɵ�һ���⻯���Ϊ������ͨʽ��CnH2n+2 �� ����C4H10������ͬ���칹�壬�ṹ��ʽ�ֱ���CH3CH2CH2CH3��CH3CH��CH3��CH3 �� ���Դ��ǣ�CnH2n+2��C4H10��CH3CH2CH2CH3��CH3CH��CH3��CH3��
��5.��������γɻ�����ΪNa2S���������ӻ�������������������ӹ��ɣ���Naԭ�ӡ�Sԭ�ӵ���ʽ��ʾ���γɹ���Ϊ ![]() ��
��
���Դ��ǣ� ![]() ��
��