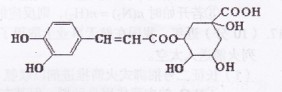��Ŀ����
��6�֣�0.2mol�л���A��0.5molO2���ܱ�������ȼ�պ�IJ���ΪCO2��CO��H2O�����ᆳ��ŨH2SO4����������10.8g;��ͨ�����ȵ�CuO��ַ�Ӧ������������3.2g;���������ͨ����ʯ�ұ���ȫ���գ���������17.6g��
��1�����ƶϸ��л���Ļ�ѧʽ��
��2����0.2mol���л���ǡ����4.6g��������ȫ��Ӧ����ȷ��A�Ľṹ��ʽ��
��3����д��A�IJ�������Ʒ�Ӧ��ͬ���칹�塣
��1�����ƶϸ��л���Ļ�ѧʽ��
��2����0.2mol���л���ǡ����4.6g��������ȫ��Ӧ����ȷ��A�Ľṹ��ʽ��
��3����д��A�IJ�������Ʒ�Ӧ��ͬ���칹�塣
��1��C2H6O , ��2��CH3CH2OH ����3��CH3OCH3 ��
��

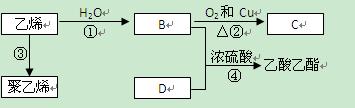
��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
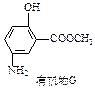
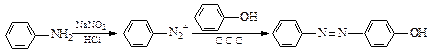
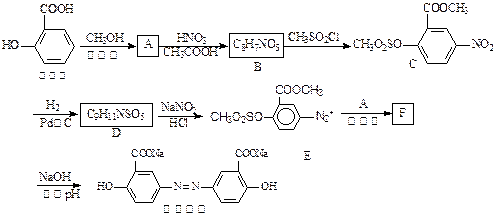
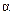 �������ᣬ����FeCl3������ɫ��Ӧ��������й���6�ֻ�ѧ������ͬ��Hԭ�ӡ�X�Ľṹ��ʽΪ________________
�������ᣬ����FeCl3������ɫ��Ӧ��������й���6�ֻ�ѧ������ͬ��Hԭ�ӡ�X�Ľṹ��ʽΪ________________
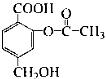 ������1 mol�û����ﷴӦ��NaOH�����ʵ�����
������1 mol�û����ﷴӦ��NaOH�����ʵ�����